Figure 5.
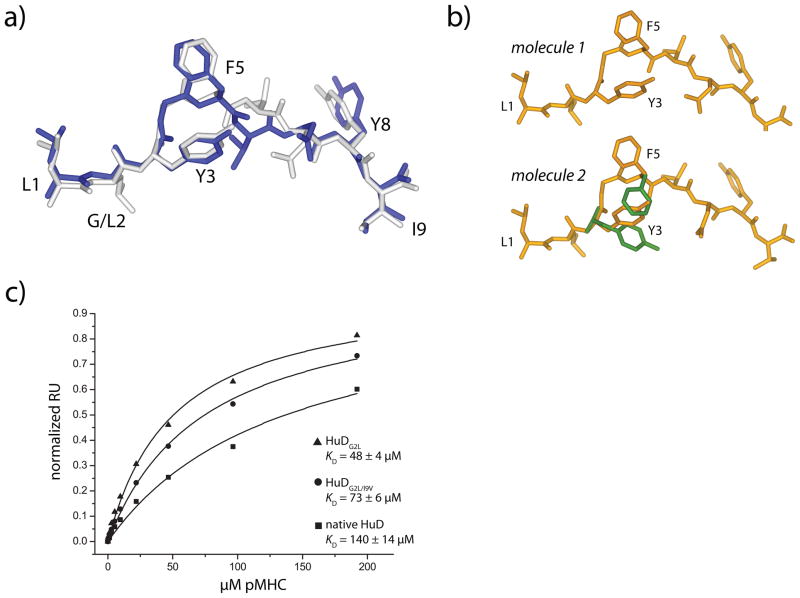
Anchor modification in the HuD peptide reveals side chain conformational differences as a component of weaker TCR affinity. A) The conformation of the peptide in the HuDG2L/HLA-A2 complex is identical to the conformation of the peptide in the ternary complex with the native, unmodified HuD peptide. The anchor-modified peptide is white, and the native peptide from the ternary complex is dark blue. B) The peptide in the first molecule in the asymmetric unit of the doubly-modified G2L/I9V HuD/HLA-A2 complex adopts the conformation seen in the complex of the native peptide with the A6 TCR (top), whereas the peptide in the second molecule in the asymmetric unit could be refined in both the conformation seen when the native peptide is TCR-bound and when the native peptide is TCR-free (alternate positions in green). C) Surface plasmon resonance indicates that the affinity of the A6 TCR is higher for the anchor-modified peptide/HLA-A2 complexes than it is for the native HuD/HLA-A2 complex, consistent with the structural results showing anchor-modification biases the side chain conformational equilibrium towards a binding-competent state.