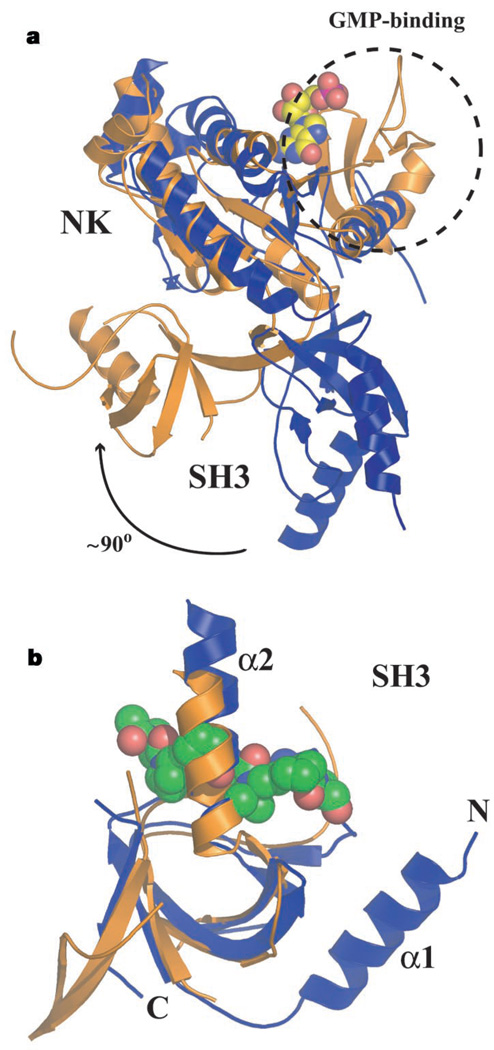
Figure 2.
Structural comparisons between PSD-95 (gold) and CaVβ2a (blue). a, Superposition of CaVβ2a and PSD-95 nucleotide kinase domains (RMSDCα = 3.9 Å). The dashed circle indicates the guanosine-monophosphate (GMP)-binding domain present in PSD-95 but absent in CaVβ2a. The guanosine monophosphate molecule bound to PSD-95 is displayed in space-filling representation. Nucleotide kinase (NK) and SH3 domains are indicated. The relative change in SH3 domain orientation is indicated. b, Superposition of PSD-95 and CaVβ2a SH3 domains (RMSDCα = 1.6 Å). Position of the polyproline ligand from a superposition with the Sem5 SH3 domain (Protein Data Bank code 2SEM) (RMSDCα = 1.8 Å) is shown in space-filling representation. The Sem5 SH3 is not shown. The DALI server generated the superpositions (http://www.ebi.ac.uk/dali/).