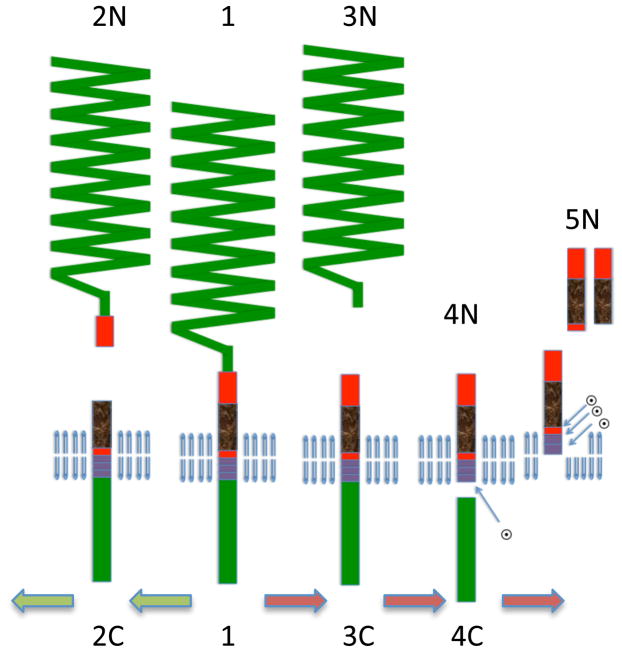
Figure 1. Pathways of APP proteolysis.
The full-length APP is a type-I integral membrane protein. It is cleaved in the exocytoplasmic domain at the start of Aβ by β-secretase and between residues 16-17 of Aβ by α secretase to generate secreted derivatives sAPPβ and sAPPα and C-terminal fragments CTFβ and CTFα. γ-Secretase cleaves CTFβ at the membrane cytoplasm boundary (ε site) to CTFγ and Aβ49, and subsequently to Aβ40 and Aβ42. Most secreted Aβ ends at residue 40 but a small percentage is longer terminating at residues 42 and 43. CTFα is similarly processed by γ-secretase (not shown). The predicted CTFγ is not seen probably due to its rapid turnover in the cell.