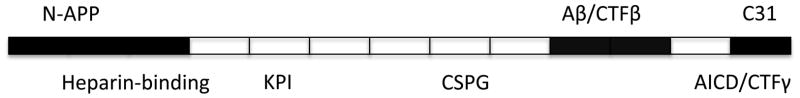
Figure 3. Multiple toxic domains and metabolites of APP have been defined.
Multiple cleavage sites have been defined on APP and these appear to generate a number of toxic proteins such as n-APP, Aβ and C31 shown above the APP molecule in the schematic diagram (not to scale). Even the major cleavage products such as CTFγ/AICD have been defined as toxic in animal models. These potentially toxic fragments demonstrate that the toxicity of APP may arise due to improper turnover and cleavage rather than due to the normal turnover pathways and argue against disrupting normal turnover mechanisms. APP is an important multifunctional protein with the key known functional domains shown below the molecule.