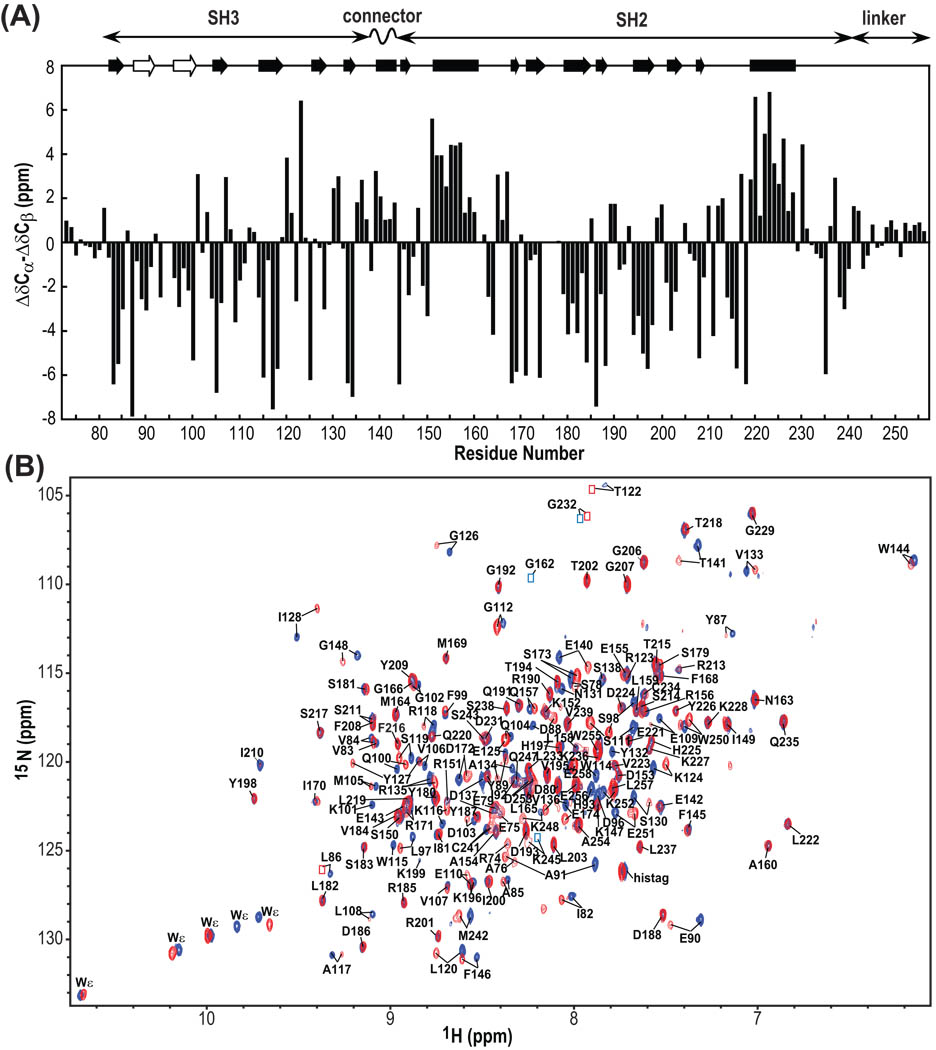
Figure 3.
13C secondary chemical shifts of Hck32L (A) and 900MHz TROSY spectra of free and Hck32L-complexed Nef (B). (A) Secondary chemical shifts (ΔCα–ΔCβ). At the top, helical and sheet segments that were identified from the crystal structure of a down-regulated full-length Hck (1AD5) as well as from our NMR work with Hck32L in solution (this study) are depicted by solid rectangles and arrows, respectively. The beta strands that are detected only in our NMR study of Hck32L are shown with open arrows. The domain locations of Hck32L are shown at the top. (B) Superposition of the 900MHz TROSY spectra of 2H/13C/15N Hck32L in the absence (blue) and presence (red) of unlabeled Nef. Assigned resonances are labeled with residue name and number. Resonances that are too weak for detection at this contour level, but clearly visible at lower levels, are indicated by blue (free Hck32L) and red (Nef-bound Hck32L) boxes.