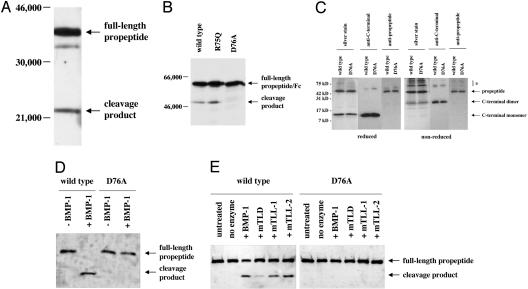
Fig. 1.
Cleavage of the myostatin propeptide by the BMP-1/TLD family of proteinases. (A and B) Detection of a propeptide degradation product in CHO cell-conditioned media. Conditioned media prepared from CHO cells expressing the propeptide (A) or WT and mutant forms of propeptide/Fc fusion proteins (B) were analyzed by SDS/PAGE followed by Western blot analysis using antibodies directed against either the myostatin propeptide (A) or IgG (B). Note that mutation of D76 to A resulted in the absence of the degradation product. (C) Purification of WT and mutant propeptide/C-terminal dimer complexes. Protein complexes were purified as described (9) and analyzed by SDS/PAGE in the presence or absence of 2-mercaptoethanol followed by Western blot analysis, as indicated. Note that, like the WT propeptide, the D76A mutant propeptide purified in a complex with the C-terminal dimer. The propeptide degradation product did not copurify with the C-terminal dimer and was thus not part of the complex. Bands denoted by * indicate misfolded myostatin species, which were evident under nonreducing conditions. (D and E) Cleavage of the propeptide by BMP-1/TLD proteinases. WT and mutant complexes were incubated with purified proteinases (14) and subjected to SDS/PAGE followed by Western blot analysis using antibodies directed against the propeptide. Incubations were carried out with 1 μg of latent complex and 250 ng of proteinase for 16 h at 37°C, except that in D the samples were incubated with an additional 250 ng of BMP-1 for 4 h more. In E, lanes labeled “no enzyme” indicate samples incubated for 16 h at 37°C in the absence of enzyme. Note that all enzymes were capable of generating the cleavage product and that the D76A mutant protein was completely resistant to cleavage.