Abstract
Purpose
To demonstrate a possible role for endogenous release of nitric oxide in determining the response of water-loading on intra-renal oxygenation as evaluated by BOLD MRI.
Materials and Methods
Twelve Sprague Dawley rats (weight 344.9 ± 40.6 g) were equally divided into two groups, A & B. Water-loading was implemented by continuous infusion of hypotonic saline containing glucose (0.25% NaCl, 0.5% glucose). Rats in group A were subject to water-loading alone, while group B rats were dosed with N-Nitro-L-Arginine Methyl Ester, (L-NAME) (10.0 mg/kg) prior to water-loading. T2* weighted images of the kidneys were obtained on a Siemens 3T Verio MRI scanner using multiple gradient recalled echo (mGRE) sequence.
Results
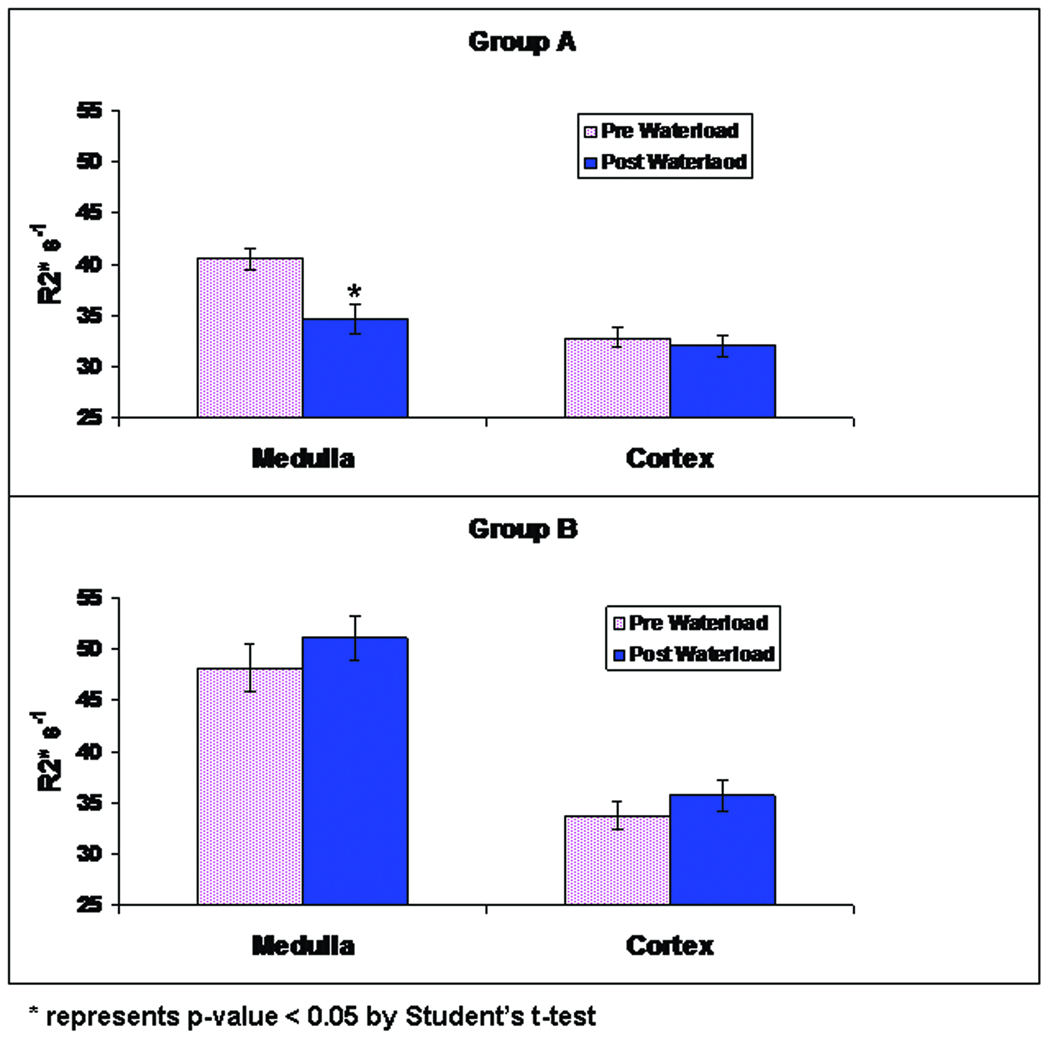
Consistent with previous reports, group A exhibited a significant decrease in medullary R2* during water-loading (40.64 ±1.10 s−1 to 34.68 ± 1.49 s−1, p<0.05). On the other hand in group B, there was no decrease in R2* during water-loading (48.11 ±2.38 s−1 to 51.06 ± 2.18 s−1). The increased pre-water-loading R2* is due to the pre-treatment with L-NAME (40.82 ± 3.23 s−1 to 48.11 ± 2.38 s−1, p<0.05).
Conclusions
Our data suggest for the first time a role for endogenous nitric oxide in determining the response of renal medullary oxygenation to water-loading.
Keywords: kidney, MRI, nitric oxide, water diuresis, renal medullary oxygenation
INTRODUCTION
Intra-renal oxygenation is now well accepted as an important determinant of renal physiology and pathophysiology (1–5). While the gold standard to evaluate intra-renal oxygenation remains to be microelectrode based measurements (1,2), their utility is limited to animal models. Blood oxygenation level dependent (BOLD) MRI has been shown to be efficacious in evaluating intra-renal oxygenation both in humans (6–9) and rodent models (10–13).
Among the several different pharmacological maneuvers investigated to-date (6,8,14), water diuresis is one of the most interesting owing to its relative non-invasive nature. While the data to-date suggest a role for prostaglandins in determining the response to water-loading on BOLD MRI (9,15), it is also suspected that nitric oxide may also play a role (16). Because there are currently no approved nitric oxide synthase (NOS) inhibitors available for human use, we have performed studies in rats.
Measurements of oxygen consumption in the human kidneys have been pursued for more than half a century. Early measurements depended on the whole organ determinations by evaluating arterio-venous difference in oxygen saturation (17). These studies demonstrated very small difference in arterio-venous oxygen saturation across the kidney. Not surprisingly, the study also demonstrated a minimal change in arterio-venous oxygen saturation following water-loading. Later measurements involving invasive microelectrodes in rat kidneys illustrated a significant gradient in tissue oxygenation within the kidney (1,2). In fact, it was suggested that kidney should be considered to be made up of two organs, the cortex and the medulla (18) based on their significant hemodynamic differences. While the cortex is supplied with blood flow far in excess of its metabolic needs, the medulla receives very little supply. Further, active transport of sodium chloride in the medullary thick ascending limbs is associated with high energy demand. Together, the medullary oxygenation is commonly described as being at hypoxic levels (3). BOLD MRI measurements inherently can differentiate the cortex and medulla and facilitate evaluation of regional changes in oxygenation in response to physiologic and/or pharmacologic maneuvers (6–9,11–14).
In this study, we hypothesized that similar to prostaglandins, bioavailability of NO is increased during water diuresis. To confirm this, similar to previous studies with cyclooxygenase (COX) inhibition (9,15,16), we have evaluated the effect of nitric oxide synthase (NOS) inhibition on subsequent water-loading.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Animal Care and Use Committee. The rats were purchased from Charles Rivers (Chicago, IL) and housed at the institutional animal care facility starting at least three days before the experiments. The rats had free access to food and water throughout the study. Twelve male Sprague Dawley rats (weight 344.9 ± 40.6 g) were divided into equal two groups randomly. Water-loading was implemented by continuous infusion of hypotonic saline containing glucose (0.25% NaCl, 0.5% glucose). Rats in group A rats were subject to water-loading alone, while group B rats were dosed with N-Nitro-L-Arginine Methyl Ester, (L-NAME) (10.0 mg/kg) prior to water-loading. There were no significant weight and age differences among groups.
On the day of experiment, rats were anesthetized with ketamine (60–100 mg/kg ip) and thiobutabarbital (Inactin, 10 mg/kg ip). A catheter (PE-50) was placed in femoral vein to induce water diuresis by infusion of hypotonic saline using an infusion pump [Genie Plus, Kent Scientific, and Litchfield, CT, USA]. In Group A, after obtaining 6 baseline BOLD MR images, animals were subject to water-loading by infusion of hypotonic saline containing glucose for 35 minute with infusion rate of 2.0ml /hour. R2* maps were obtained every 3 minutes.
In Group B, after obtaining 6 baseline images of the kidneys, a slow bolus injection of LNAME was administered through catheter and BOLD MR images were obtained with interval of 3 minute for 45 minute followed by infusion of hypotonic saline with same infusion rate as above to induce the water loading.
Imaging Protocol
MRI acquisitions were performed on a 3.0 T Verio (Siemens Medical Solutions, Erlangen, Germany) scanner using quadrature extremity coil was used for signal reception. T2* weighted images of the kidneys were obtained using multiple gradient recalled echo (mGRE) sequence with 12 echoes. Imaging parameters include: TR = 62 ms, TE = 3.1 – 44.6 ms, no of slices = 1, NEX = 20, FA = 20, slice thickness 3.0 mm, FOV = 12.0×7.5 cm, matrix 256×256, bandwidth 260 Hz/voxel.
Data Analysis
R2* (=1/T2*) measurements were performed by drawing circular regions of interest (ROI), three each on cortex and medulla, on the T2* map generated by the inline processing on the scanner. Typical ROI size was about 20 pixels and were placed based on the cortex or medulla on the anatomical image and copied to the corresponding T2* map. Statistical significance was determined by paired one tail Student’s t-test. Additionally, R2* color maps were generated offline using Matlab (Mathwork, Natick MA). All the maps were scaled from zero to 75 s−1 representing well to poorly oxygenated. An increase in R2* implies a decrease in oxygenation and vice versa.
RESULTS
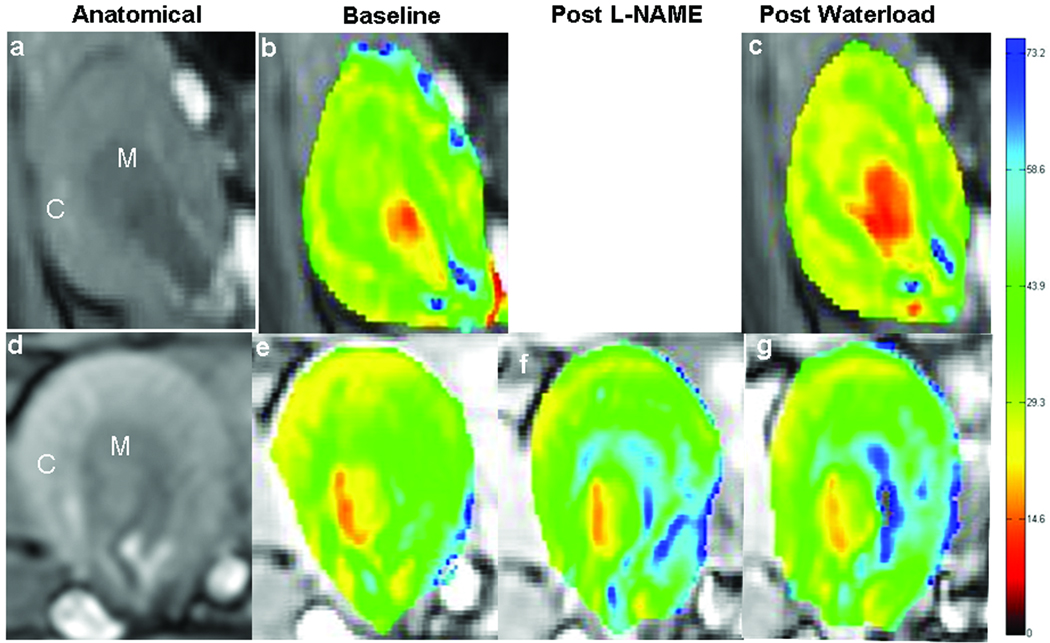
Figure 1 illustrates an anatomical and the corresponding R2* maps of a representative rat kidney from each group of animals. All the maps were scaled from 0 to 75 s−1 corresponding to the degree of oxygenation from well saturated (red) to poorly saturated (blue). While water-loading decreased medullary R2* in group A, a similar response was absent in group B. Time course of change in R2* in representative animals is illustrated in Figure 2. Note the clear decrease in medullary R2* in group A during water-loading, while a similar response is completely absent in group B. Note that the pre-water-load medullary R2* was significantly higher in this group due to the direct effect of L-NAME (14).
Figure 1.
shows R2* maps of rat kidneys in one representative animal from each of the groups A and B. The top row illustrates images from group A (water-load): (a) anatomical, (b) baseline R2* map, (c) R2* map obtained during water loading. The bottom row similarly shows images from group B: (d) anatomical, (e) baseline R2* map, R2* maps obtained (f) post L-NAME, and (g) during water loading. All the maps were scaled from zero (red) to 75 (blue) representing a range of hemoglobin oxygen saturation levels. M indicates outer medulla and C represents the cortex.
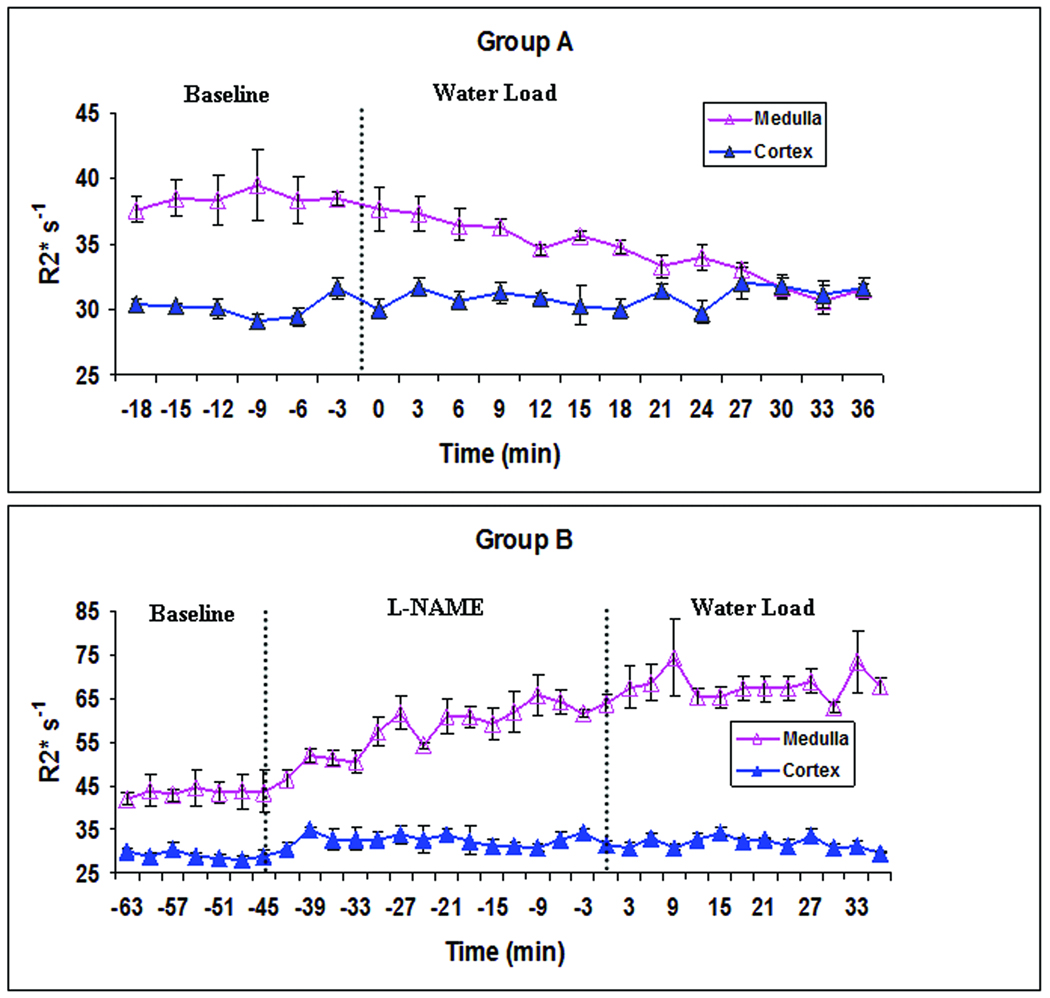
Figure 2.
Illustrates time course of cortical and medullary variation in R2* during water-loading in one representative animal in each of the groups A (top) and B (bottom). Note the significant decrease in medullary R2* values, approaching the cortical values in group A during water-loading. Note the absence of similar decrease in R2* in group B during water-loading. Also note the significant increase in R2* in group B prior to water-loading illustrating a decline in medullary oxygenation due to the administration of L_NAME. Time zero represents the start of water load in both groups of animals. The error bars show the standard error estimated over 3 ROIs in each, cortex and medulla.
Figure 3 is the summary plot showing R2* changes during water-loading in renal medulla and cortex in the two groups of animals. The medullary R2* in group A decreased significantly during the water diuresis in group A. In group B, a similar response to water-loading was completely abolished.
Figure 3.
Summary plots illustrating cortical and medullary R2* in rat kidneys during water-loading in each of the group A (top) and B (bottom). Shown are R2* values in cortex and medulla before and after 35 mins of waterloading. Note that only the decrease in medullary R2* in group A reach statistical significance following water-load with the p < 0.05 by paired one tail Student’s t-test. The error bars represent SD over all animals.
DISCUSSION
Presence of renal medullary hypoxia and its consequences to various renal diseases is well accepted now (3,5). A key corollary to the medullary hypoxia theory is that it may not be the level of hypoxia itself that is a risk factor, but rather the compromised ability to maintain renal oxygenation status that is a risk factor. Among the many hypothetical control mechanisms the role for prostaglandins and nitric oxide are better appreciated (2). The possible role of prostaglandins was previously illustrated in humans using BOLD MRI (9,15). It was shown that the medullary oxygenation was significantly improved during water-loading, but not when pretreated with COX inhibitors such as naproxen (15) and ibuprofen (9). While a similar role for nitric oxide was suspected, similar experiments could not be performed due to the lack of approved NOS inhibitors for human use. Water-loading in rodent models have not been reported extensively. It was recently shown that it is feasible to implement water-loading paradigm in rats and similar to humans an increase in medullary oxygenation during water-loading was reported (16). Our observations in this study are consistent with the previous reports in rat (16) and human kidneys (9,15) in terms of the response to water-loading. In the original studies that introduced renal BOLD MRI (8), data were acquired with both gradient echo (GRE) and spin echo (SE) to show that changes due to water-load were only apparent on the GRE and not on the SE. Future studies could incorporate techniques to allow for simultaneous measurement of R2 and R2* allowing for estimation of R2’ (the susceptibility component) similar to the one proposed by Ma and Wehrli (19). This could facilitate more straightforward interpretation of BOLD MRI data eliminating confounding effects such as due to change in water content.
In the current study, we show for the first time a possible role for nitric oxide in determining the response to water-loading on BOLD MRI. As illustrated in figures 1–3, pre-treatment with L-NAME completely abolishes the response to water-load. This is very similar to the previous observations with COX inhibition in humans (9,15). A key difference is the apparent direct effect of NOS inhibition observed in this study. At the doses of COX inhibitors and the route of administration used in the human studies (9,15), there was no measurable change in the R2* values prior to water-loading. Compared to the oral administration of COX inhibition in humans, L-NAME was administered intravenously and the dose was higher than what has been reported for investigational use in humans (14).
The role of nitric oxide in the vascular endothelium is well recognized and it is known that its production and hence bioavailability is impaired in several diseases such as hypertension, diabetes, atherosclerosis, aging etc. (20). Results from the present study suggest that we may observe a reduced response to waterload in subjects with endothelial dysfunction. Previous studies had indicated this in elderly (9) and in early diabetics (21). Alternately, one could observe a reduced response to L-NAME as shown previously (11). Endothelial dysfunction may also predispose to acute renal failure as suggested by previous studies on contrast induced nephropathy (2,22). The results may also explain mechanistically why hydration is an effective way to mitigate effects of radiocontrast, i.e. stimulating endogenous protective mechanisms such as prostaglandins and nitric oxide. Assuming that compromised ability to upregulate prostaglandins and/or nitric oxide is a risk factor for acute renal failure such as that associated with radiocontrast administration, BOLD MRI response to water-loading could potentially be used as a way of identifying subjects at such risk.
In conclusion, similar to prostaglandins, nitric oxide may also play a key role in determining the renal medullary oxygenation status during water-loading. This could further support the recent strategy to combine NO donation with COX-inhibitors for better pain management with reduced nephrotoxic risk associated with COX inhibition (16).
Acknowledgments
Work supported in part by a grant from the National Institutes of Health, R01-DK053221
REFERENCES
- 1.Aukland K, Krog J. Renal oxygen tension. Nature. 1960;188:671. doi: 10.1038/188671a0. [DOI] [PubMed] [Google Scholar]
- 2.Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94(3):1069–1075. doi: 10.1172/JCI117421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brezis M, Rosen S. Hypoxia of the renal medulla--its implications for disease. N Engl J Med. 1995;332(10):647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 4.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol. 1994;267(6 Pt 2):F1059–F1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 5.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1):17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann L, Simon-Zoula S, Nowak A, et al. BOLD-MRI for the assessment of renal oxygenation in humans: acute effect of nephrotoxic xenobiotics. Kidney Int. 2006;70(1):144–150. doi: 10.1038/sj.ki.5000418. [DOI] [PubMed] [Google Scholar]
- 7.Li LP, Halter S, Prasad PV. Blood oxygen level-dependent MR imaging of the kidneys. Magn Reson Imaging Clin N Am. 2008;16(4):613–625. doi: 10.1016/j.mric.2008.07.008. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94(12):3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 9.Prasad PV, Epstein FH. Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int. 1999;55(1):294–298. doi: 10.1046/j.1523-1755.1999.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.dos Santos EA, Li LP, Ji L, Prasad PV. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest Radiol. 2007;42(3):157–162. doi: 10.1097/01.rli.0000252492.96709.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Storey P, Kim D, Li W, Prasad P. Kidneys in hypertensive rats show reduced response to nitric oxide synthase inhibition as evaluated by BOLD MRI. J Magn Reson Imaging. 2003;17(6):671–675. doi: 10.1002/jmri.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li LP, Ji L, Lindsay S, Prasad PV. Evaluation of intrarenal oxygenation in mice by BOLD MRI on a 3.0T human whole-body scanner. J Magn Reson Imaging. 2007;25(3):635–638. doi: 10.1002/jmri.20841. [DOI] [PubMed] [Google Scholar]
- 13.Priatna A, Epstein FH, Spokes K, Prasad PV. Evaluation of changes in intrarenal oxygenation in rats using multiple gradient-recalled echo (mGRE) sequence. J Magn Reson Imaging. 1999;9(6):842–846. doi: 10.1002/(sici)1522-2586(199906)9:6<842::aid-jmri12>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li LP, Ji L, Santos EA, Dunkle E, Pierchala L, Prasad P. Effect of nitric oxide synthase inhibition on intrarenal oxygenation as evaluated by blood oxygenation level-dependent magnetic resonance imaging. Invest Radiol. 2009;44(2):67–73. doi: 10.1097/RLI.0b013e3181900975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumkur SM, Vu AT, Li LP, Pierchala L, Prasad PV. Evaluation of intra-renal oxygenation during water diuresis: a time-resolved study using BOLD MRI. Kidney Int. 2006;70(1):139–143. doi: 10.1038/sj.ki.5000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji LL, Schnitzer T, Du H, Prasad PV. Intra-renal oxygenation in rat kidneys during water loading: effects of cyclooxygenase (COX) inhibition and nitric oxide (NO) donation. J Magn Reson Imaging. 2010;32(2):383–387. doi: 10.1002/jmri.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark JK, Barker HG. Studies of renal oxygen consumption in man. I. The effect of tubular loading (PAH), water diuresis and osmotic (mannitol) diuresis. J Clin Invest. 1951;30(7):745–750. doi: 10.1172/JCI102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein FH, Agmon Y, Brezis M. Physiology of renal hypoxia. Ann N Y Acad Sci. 1994;718:72–81. discussion 81-72. [PubMed] [Google Scholar]
- 19.Ma J, Wehrli FW, Song HK, Hwang SN. A single-scan imaging technique for measurement of the relative concentrations of fat and water protons and their transverse relaxation times. J Magn Reson. 1997;125(1):92–101. doi: 10.1006/jmre.1996.1086. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz BG, Economides C, Mayeda GS, Burstein S, Kloner RA. The endothelial cell in health and disease: its function, dysfunction, measurement and therapy. Int J Impot Res. 22(2):77–90. doi: 10.1038/ijir.2009.59. [DOI] [PubMed] [Google Scholar]
- 21.Epstein FH, Veves A, Prasad PV. Effect of diabetes on renal medullary oxygenation during water diuresis. Diabetes Care. 2002;25(3):575–578. doi: 10.2337/diacare.25.3.575. [DOI] [PubMed] [Google Scholar]
- 22.Prasad PV, Priatna A, Spokes K, Epstein FH. Changes in intrarenal oxygenation as evaluated by BOLD MRI in a rat kidney model for radiocontrast nephropathy. J Magn Reson Imaging. 2001;13(5):744–747. doi: 10.1002/jmri.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]