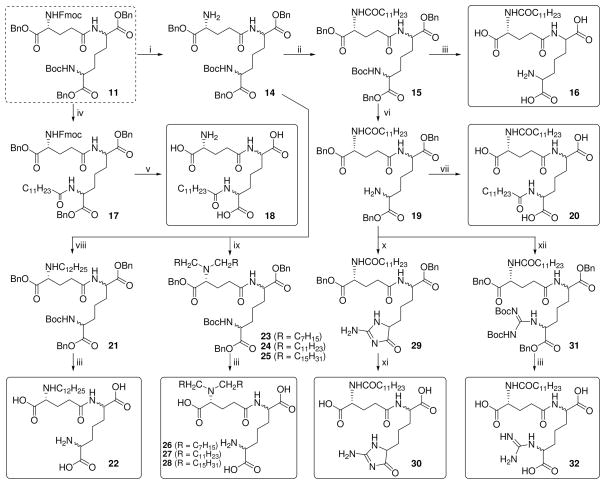
Scheme 2.
Reagents: i. 30% Piperidine, CH2Cl2; ii. C11H23COCl, pyridine, DMAP; iii. (a) H2, Pd(OH)2/C, MeOH, 60 psi (b) CF3COOH; iv. (a) CF3COOH (b) C11H23COCl, TEA, CH2Cl2; v. H2, Pd(OH)2/C, AcOH, MeOH, 60 psi; vi. CF3COOH; vii. (a) C11H23COCl, pyridine, CH2Cl2 (b) H2, Pd(OH)2/C, MeOH, 60 psi; viii. C11H23CHO (1eq.), MP-CNBH3, CH2Cl2, MeOH, AcOH; ix. RCHO(excess), MP-CNBH3, CH2Cl2, MeOH, AcOH; x. 1H-Pyrazole-1-carboxamidine.HCl, pyridine, MW irradiation, 60°C; xi. H2, Pd(OH)2/C, MeOH, 60 psi; xii. N,N′-Di-Boc-1HPyrazole- 1-carboxamidine•HCl, pyridine, THF, 50°C.