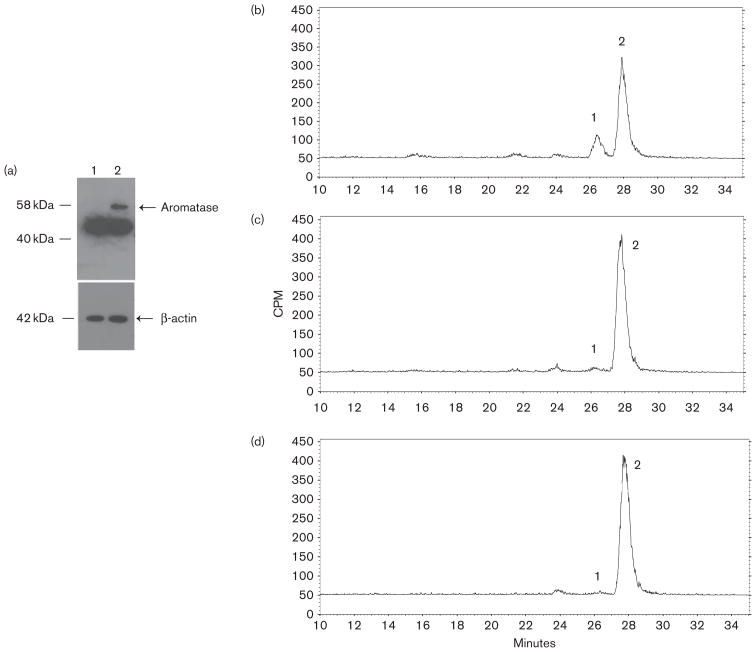
Fig. 3.
Aromatase expression in HEK293-aro cells and aromatase-inhibiting activity of exemestane versus 17-dihydroexemestane in HEK293-aro cell homogenates. Panel (a), Western blot analysis of aromatase protein expression was performed using lysates from the aromatase-over-expressing HEK293 cell line used for the aromatase activity assays described in this study. Lane 1, parent HEK293 cell lysate (40 μg total protein); lane 2, HEK293-aro cell lysate (40 μg total protein). Panel (b), Estrone formation in HEK293-aro cells+5 μmol/l androst-[4-14C]-ene-3,17-dione. Panel (c), Inhibition of estrone formation in HEK293-aro cells+5 μmol/l androst-[4-14C]-ene-3,17-dione by 3 μmol/l 17-dihydroexemestane. Panel (d), Inhibition of estrone formation in HEK293-aro cells+5 μmol/l androst-[4-14C]-ene-3,17-dione by 3 μmol/l exemestane. For panels b–d, aromatase activity was determined using HEK293-aro cell homogenates (125 μg) and quantified by radioflow-HPLC as described in the Materials and methods section. Aromatase activity was determined by measuring the formation of 14C-labeled estrone from androst-[4-14C]-ene-3,17-dione, the major endogenous substrate for aromatase. Peak 1, estrone; peak 2, androst-[4-14C]-ene-3,17-dione. No estrone formation was observed using (– minus aromatase) HEK293 cell homogenates (data not shown).