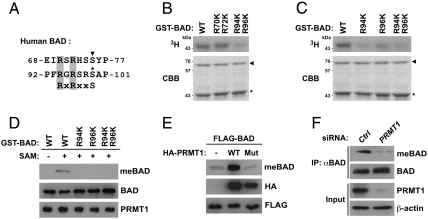
Fig. 2.
PRMT1 methylates BAD at Arg-94 and Arg-96 in vitro and in vivo. (A) Alignment of the human BAD sequence with the Akt consensus phosphorylation motif (RxRxxS). The corresponding arginine residues are shaded in gray. Two known phosphorylation sites of PKA/RSK and Akt are shown by the arrowhead and asterisk, respectively. (B and C) A series of GST-BAD point mutants in which one or two arginine residues were substituted with lysine were incubated with GST-PRMT1 in the presence of [3H]SAM. Reaction products were analyzed by autoradiography (Upper) and Coomassie brilliant blue staining (Lower). The arrowhead and asterisk indicate total amounts of GST-PRMT1 and GST-BAD, respectively. (D) A series of GST-BAD point mutants in which one or two arginine residues were substituted with lysine were incubated with GST-PRMT1 in the presence or absence of SAM. Reaction products were analyzed by immunoblotting with anti-MeBAD, anti-BAD, or anti-PRMT1 antibodies. (E) HEK293T cells were transfected with FLAG-BAD with or without wild-type or G98R mutant of HA-PRMT1. Whole-cell lysates were analyzed by immunoblotting with anti-MeBAD, anti-HA, or anti-FLAG antibodies. (F) Whole-cell lysates from HEK293T cells transfected with control (Ctrl) or PRMT1-specific siRNA were immunoprecipitated with anti-BAD antibody, followed by immunoblotting with anti-MeBAD or anti-BAD antibodies.