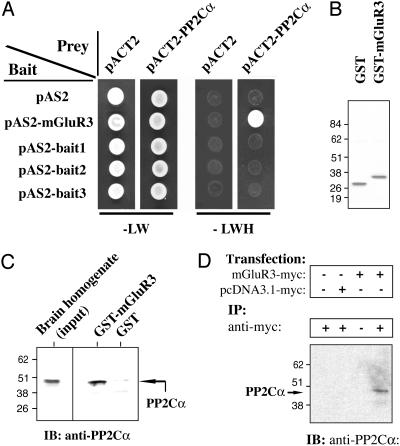
Fig. 1.
Interaction of PP2Cα with mGluR3 in a yeast two-hybrid assay (A), in a GST pull-down assay (B and C), and by coimmunoprecipitation (D). (A)Onthe left, bait constructs are indicated (pAS2 corresponds to the empty-bait plasmid). On the top, prey constructs are indicated (pACT2 corresponds to the empty-prey plasmid). Double transformants were spotted and grown for 3 days at 30°C on -LW medium (Left)oron -LWH medium (Right) to check for histidine auxotrophy. All the clones grew on -LW medium (growth control), but only coexpression of pAS2-mGluR3 and pACT2-PP2Cα allowed growth on histidine-dropout plates. (B) GST or GST-mGluR3-830-879 (last 50 C-terminal amino acids of mGluR3 fused to the GST domain) were expressed in E. coli, purified, and analyzed by SDS/PAGE and staining with Coomassie blue. (C) Total brain homogenate (2 mg) was incubated with immobilized GST (negative control) or GST-mGluR3-830-879 (GST-mGluR3). Retained proteins were analyzed by SDS/PAGE and immunoblotting (IB) by using an anti-PP2Cα antibody. Brain homogenate input (50 μg) was also analyzed (Left). (D) mGluR3-myc or the empty plasmid (pcDNA3.1-myc) was expressed in Cos7 cells. Cells were lysed and immunoprecipitation (IP) was carried out by using a monoclonal anti-myc antibody. Proteins were analyzed by SDS/PAGE and immunoblotting with anti-PP2Cα antibody.