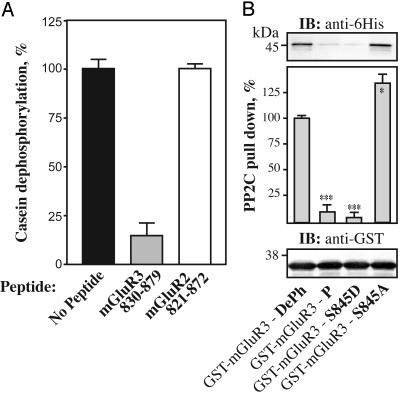
Fig. 5.
Characterization of the interaction of the dephospho and phospho forms of the mGluR3 cytoplasmic tail with PP2Cα. (A) Positive control (black bar), dephosphorylation of [32P]casein by PP2Cα in the absence of competing polypeptide; mGluR3-830-879 (gray bar), dephosphorylation of [32P]casein (3 μM) by PP2Cα in the presence of 3 μM of mGluR3-830-879; negative control (white bar), dephosphorylation of [32P]casein (3 μM) by PP2Cα in the presence of 3 μM mGluR2-821-872 prepared in the same way as the mGluR3 polypeptide. Data for three experiments (mean ± SEM). (B) 6His-PP2Cα (1 μg) was incubated with 10 μg of immobilized GST-mGluR3-830-879 fusion proteins (GST-mGluR3-wt, no modification; GST-mGluR3-P, Ser-845 phosphorylated in vitro by PKA; GST-mGluR3-S845D and GST-mGluR3-S845A, mutation of Ser-845 to Asp and Ala, respectively). Retained proteins were analyzed by SDS/PAGE and immunoblotting by using an anti-6His antibody. Results were normalized after immunoblotting with an anti-GST antibody. Data for three experiments (mean ± SEM) were compared with the values obtained for the wild-type DePh mGluR3 (*, P < 0.5; ***, P < 0.001, Student's t test).