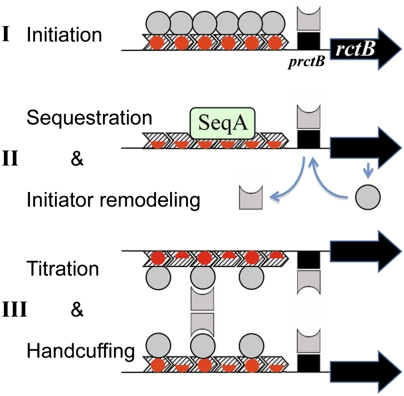
Fig. 6.
A model of chrII replication cycle. For simplicity, the incII region is omitted from the diagrams. (I, Top) Initiation requires RctB initiators (circles) to saturate the origin iterons. The iterons bind only when they are fully methylated (marked by red circles). RctB changes form (circle to square with one semicircular edge) when it serves as an autorepressor by binding to a 39-mer–like sequence (black rectangle). (II, Middle) Iterons get hemimethylated (marked by red semicircles) following initiation, and become substrates for SeqA binding. The 39-mer remains binding competent and maintains autorepression throughout the cell cycle. The basal level of synthesis accumulates RctB in the remodeled form due to interactions from the three 39-mers of the origin region. (III, Bottom) As the iterons become fully methylated, they regain RctB binding proficiency. Initiation is prevented by distribution of the initiators to the two sister origins, so that none can be saturated (titration), and handcuffing of the sister origins. The handcuffing is mediated proficiently by the remodeled RctB, which serves as a bridging molecule. The tetrameric protein bridge is invoked because this is believed to be the case in handcuffing of plasmids (8). The RctB-bound iterons and 39-mers also handcuff directly (heterohandcuffing; Fig. 5). Later in the cell cycle, unmodified RctB dominates and eventually saturates the origin, causing initiation to ensue. In this model, the change in proportion of two forms of the initiator at different stages of the cell cycle is central to the replication switch.