Fig. 1.
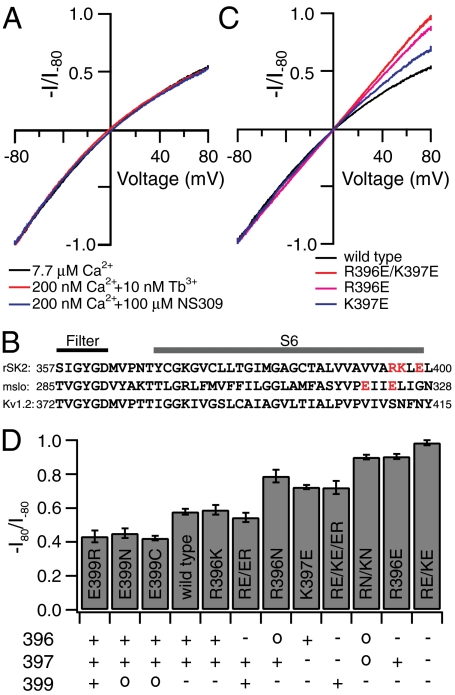
Charged residues near the inner mouth influence the intrinsic rectification of SK channels. (A). Representative SK currents in response to a voltage ramp from -80 mV to 80 mV, recorded under inside-out patch clamp configuration, are normalized to the current level at -80 mV to compare inward rectification. SK currents are activated by 7.7 μM Ca2+ (chelated with 5 mM HEDTA) (black), by 10 nM Tb3+ added to the Chelex-100 column-treated chelator-free internal solution (contaminating Ca2+ approximately 200 nM) (red), or by 200 nM Ca2+ (chelated with 5 mM EGTA) in the presence of 100 μM NS309 (blue). (B). Alignment of the sequence in the S6 domain between SK (rSK2), BK (mslo), and Kv1.2 K+ channels. Charged residues of interest in SK and BK channels are highlighted in red. (C). Representative SK currents activated by 7.7 μM Ca2+ for R396E/K397E (red), R396E (purple), and K397E channels (blue) are normalized and plotted together to compare inward rectification with wild type (black). (D). The average levels of inward rectification, characterized by the ratio between current amplitude at 80 mV and that at -80 mV (-I80/I-80), were determined from three to six patches for each type of mutant or wild-type channel. Mean value ± SD is plotted for each construct. Expected charges at positions 396, 397, and 399 for wild-type and mutant channels are shown for each construct below the bar graph.