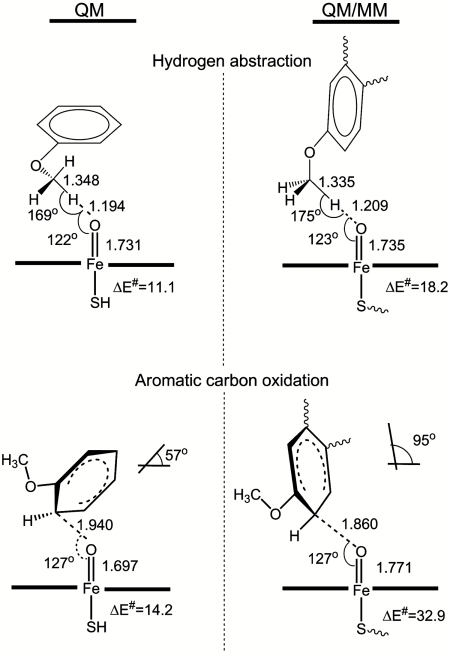
Fig. 1.
Calculated TS structures and activation energies (at the B3LYP/BSI//B3LYP/LACVP** level, including ZPE) in QM and QM/MM calculations for quartet state hydrogen abstraction from the O-methyl group of dextromethorphan and doublet state C2 aromatic hydroxylation. The angle between the best-fit planes for the aromatic ring and the heme group is shown for the aromatic oxidation TS structures.