Fig. 2.
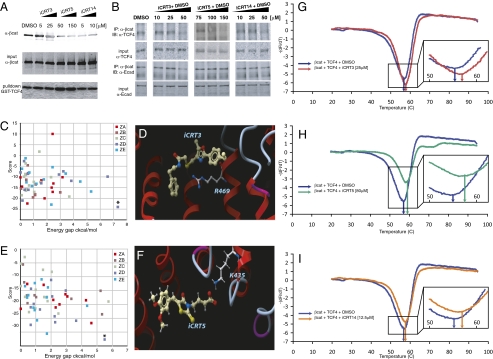
(A) Effect of candidate compounds on the interaction of purified β-cat-His and GST-TCF4. iCRT3, -5, and -14 show a significant inhibitory effect on these interactions compared with nontreated (NT) and DMSO-treated binding reactions. Bottom shows comparable amounts of GST-TCF4 being pulled down. (B) Effect of candidate compounds on β-cat's interaction with endogenous TCF4. HEK293 cells were transfected with S37Aβ-cat and treated overnight with candidate compounds at the indicated concentrations. β-cat immunoprecipitates from whole-cell lysates blotted for endogenous TCF4 show significant reduction in β-cat-TCF4 interaction in the presence of compounds while having negligible effect on β-cat-E-cad interaction. Images are representative of independent experiments and are from different gels. (C and E) Colored squares represent the energy scores (y axis) of the best alternative conformations that were accepted during the flexible Monte Carlo docking simulation for iCRT3 (C) and -5 (E) against each of the 20 pockets on the different crystal structures of β-cat (different colors for different Protein Data Bank crystal structures). Asterisk represents the docked conformation with the best energy score, which for these two compounds, is the lowest energy and is clearly distinguishable (significant energy gap; x axis) from nearby conformations. (D and F) Docked conformations of iCRT3 (D) and -5 (F) in the pocket lined by R469 and K435 on β-cat. (G–I) Traces showing negative derivatives of changes in fluorescence of β-cat-TCF4-SYPRO Orange complex in the presence of indicated concentrations of iCRT3 (red trace in G), -5 (green trace in H), or -14 (orange trace in I). Note the shift in the melting temperature (Tm indicated by arrows; G Inset, H Inset, and I Inset) in presence of iCRTs compared with that in the presence of DMSO (blue trace in G–I).