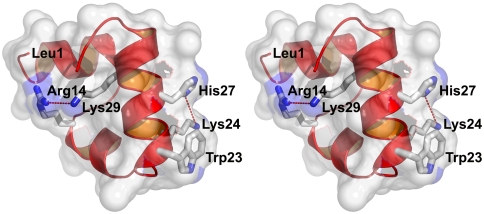
Fig. 1.
X-ray structure of villin headpiece subdomain, HP35(His27), (Protein Data Bank ID code 1YRF) showing key residues (sequence: LSDED FKAVF GMTRS AFANL PLWKQ QNLKK EKGLF) (23). Trp23 is the fluorescence probe; His27 replaces Asn27 of the wild-type sequence, and when protonated reduces the fluorescence of Trp23 upon folding; Lys24 and Lys29 make repulsive electrostatic interactions with protonated His27 and Arg14, respectively. The ϵ-amino nitrogen of Lys24 is 6.1 Å from the nearest protonated nitrogen of the imidazole ring of His27 and the ϵ-amino nitrogen of Lys29 is 5.3 Å from the nearest charged amino atom of Arg14. Removal of the ϵ-amino nitrogen groups of Lys24 and Lys29 to form norleucines in the mutant called HP35(Nle24,His27,Nle29) eliminates the repulsive interaction, thereby stabilizing the protein and increasing the folding rate (24, 25).