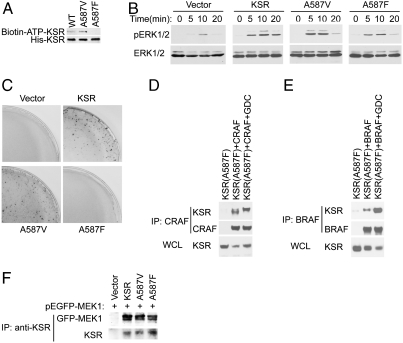
Fig. 2.
The ability of KSR to bind ATP is required for the function of KSR. (A) Mutagenesis was used to substitute phenylalanine or valine for A587 of mouse 6xHis-KSR1. Each mutant was expressed in cells, purified using Ni2+-agarose and tested for ATP binding using a biotinylated-ATP analog after UV cross-linking and immunoblotting for the presence of biotin. (B) KSR deficient fibroblasts were reconstituted with YFP fused to wild-type or mutated KSR and sorted to generate cell lines with similar expression levels (see Supporting Information). Cells were stimulated with EGF for the indicated times and cell lysates were immunoblotted with an antibody to phosphorylated ERK (pERK). (C) Stably transfected KSR deficient cell lines, described in B, were transfected with an expression vector for RASV12 and assessed for transformed colony formation. (D) Constitutive KSR/CRAF complexes induced by A587F. FLAG-KSR immunoprecipitates were prepared from lysates from cells expressing A587F FLAG-KSR with myc-CRAF and immunoblotted for CRAF (myc). This experiment was performed with Fig. 1D and is directly comparable. The complete blot is shown in Supporting Information. (E) A587F KSR mutation does not affect basal binding to BRAF. Experiment was performed as described in E, except BRAF was used instead of CRAF. (F) A587F KSR mutation does not affect MEK binding. KSR immunoprecipitates from cell coexpressing GFP-MEK1 and WT or KSR mutants were immunoblotted for GFP-MEK and KSR (FLAG).