ABSTRACT
Purpose: The primary purpose of this study was to determine the extent to which health factors, functional measures, and pulmonary impairment explain performance on 6-Minute Walk Test (6MWT) distance in ambulatory persons with multiple sclerosis (MS). Another purpose was to determine the effect of disability and age on 6MWT performance and explanatory factors.
Methods: A cross-sectional study design was used to evaluate factors that explain performance on the 6MWT in 64 community-dwelling persons with MS-related disability (Expanded Disability Status Scale [EDSS] 3.8±1.6). Of the 64 participants, 43 (67.2%) exhibited mild disability (EDSS <4.0) and 21 (32.8%) had moderate disability (EDSS 4.0–6.5). A regression analysis compared 6MWT performance to measures of health factors (EDSS, number of medications, number of comorbidities, resting HR, systolic and diastolic blood pressure [BP]); physical performance (functional stair test [FST], sit-to-stand test [SST], static standing balance [BAL], Fatigue Severity Scale [FSS], Activities-specific Balance Confidence [ABC] Scale); and pulmonary function (forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC], maximal voluntary ventilation [MVV], maximal inspiratory pressure [MIP], maximal expiratory pressure [MEP]).
Results: EDSS, ABC, FST, SST, BAL, MVV, MIP, and MEP were significantly associated with 6MWT distance after adjusting for age. Multiple step-wise linear regression analysis revealed that ABC, FST, and BAL were significant and independent explanatory factors of 6MWT distance. ABC and FST explained 75% of the variance in 6MWT performance (R2=0.75). Curvilinear regression analysis revealed that the FST is the most significant explanatory factor for 6MWT distance, explaining 79% of the variance (R2=0.79).
Conclusions: 6MWT performance in persons with MS was explained by balance confidence (ABC) and stair-climbing ability (FST). The ABC and FST may be practical clinical measures for explaining walking ability and determining risk for disablement in persons with MS.
Key Words: fatigue, multiple sclerosis, muscle strength, postural balance, 6MWT, walking
RÉSUMÉ
Objectif : L'objectif principal de cette étude était de déterminer jusqu'à quel point les facteurs de santé, les mesures fonctionnelles et les incapacités pulmonaires influent sur les résultats en terme de distance au test de marche de 6 minutes (6MWT) chez les personnes qui souffrent de sclérose en plaques (SP) et qui sont encore ambulatoires. Un autre objectif consistait à évaluer l'effet de l'incapacité et de l'âge sur les résultats au 6MWT et sur les facteurs explicatifs.
Méthode : Une étude transversale a été utilisée pour évaluer les facteurs expliquant les résultats au 6MWT chez 64 personnes avec SP qui vivent dans des habitations communautaires, avec incapacités liées à leur maladie (échelle d'incapacité EDSS 3,8±1,6). Au total, 43 sujets (67,2 %) démontraient une incapacité légère (EDSS<4,0) et 21 (32,8 %) avaient une incapacité modérée (EDSS 4,0–6,5). Une analyse de régression a permis de comparer le 6MWT aux mesures des facteurs de santé (EDSS, nombre de médicaments, nombre de comorbidités, FC au repos, tension artérielle systolique et diastolique [TA]; performance physique [test d'escalier ou FST], test assis-debout [SST], test d'équilibre statique [BAL], échelle de la fatigue [FSS], échelle ABC (échelle d'équilibre en activité spécifique] et fonction pulmonaire (volume expiratoire forcé en 1 seconde ou FEV1], capacité vitale forcée [FVC], ventilation volontaire maximale [MVV], pression inspiratoire maximale [MIP], pression expiratoire maximale [MEP]).
Résultats : Les tests EDSS, ABC, FST, SST, BAL, MVV, MIP et MEP étaient associés de manière significative à la distance parcourue lors du 6MWT, après ajustement de l'âge. Une analyse de régression linéaire multiple progressive a révélé que l'ABC, le FST et le BAL constituaient des facteurs explicatifs indépendants significatifs de la distance parcourue au 6MWT. L'ABC et le FST expliquaient 75 % de la variation de la performance au 6MWT (R2=0,75). Une analyse de régression curviligne a permis de constater que le FST est le facteur explicatif le plus important pour la distance du 6MWT, expliquant 79 % de la variation (R2=0,79).
Conclusions : La performance au 6MWT chez les personnes souffrant de SP s'explique par la confiance à l'équilibre (ABC) et la capacité à monter un escalier (FST). L'ABC et le FST pourraient être des mesures pratiques cliniques pour expliquer la capacité à marcher, et déterminer les risques d'éventuelles incapacités dans les cas de SP.
Mots clés : équilibre postural, fatigue, force musculaire, marche, sclérose en plaques, test de marche de 6 minutes (6MWT)
INTRODUCTION
Multiple sclerosis (MS) is a primary disorder of the central nervous system affecting neural centres and motor pathways and resulting in muscle weakness, fatigue, balance and gait dysfunction, and pulmonary impairment.1–4 Functional deficits and impairments, such as muscle weakness, fatigue, and impaired ventilation, have long been recognized as major causes of morbidity and mortality in individuals with advanced MS.5,6 Walking and stair climbing are critically important to maintaining community-level functioning and independence.7 Tests of lower-extremity function are known to be strong predictors for risk of disability and hospitalization in older community-dwelling persons;8–11 however, there are limited data on physical performance function in community-dwelling individuals with MS. Determining factors that contribute to limited walking ability in people with MS will assist therapists in planning treatment for these individuals.
Originally developed to assess disability in patients with chronic obstructive lung disease, the 6-minute walk test (6MWT) has since been extensively studied and used with older persons and persons with heart failure, pulmonary hypertension, cardiovascular conditions, stroke, obesity, human immunodeficiency virus, acquired brain injury, adult cerebral palsy, and MS.12–27 Reference values for the 6MWT are reported for people aged 20 to 5015 and 55 to 75 years.16 Performance on the 6MWT is influenced by many factors, including age,16,19,20,22 gender,20 race,20 height,20 weight,17,20 health condition (medications,14 body mass index [BMI],17 resting heart rate [HR]), physical parameters14 (strength, balance, speed, sensation, pain, forced expiratory volume in 1 second [FEV1]16,20), and psychological conditions (depression, cognition, affect).13,14,20 Camarri et al. found that in healthy adults, height and FEV1 predicted 33.9% of the variance in distance walked on the 6MWT.16 Enright and Sherrill predicted 40% of the variance in 6MWT performance among healthy adults using height, weight, and age.19
Performance on the 6MWT is significantly reduced in persons with MS, which may suggest an increased risk of mortality and limited mobility.24,28,29 A reduced distance on the 6MWT has predicted increased risk of morbidity and mortality in several patient populations.13,24–27 Therefore, identifying factors that influence 6MWT performance in persons with MS is important. Research examining factors related to 6MWT in persons with MS is very limited. Recently, Savci et al. (2005) reported 6MWT in persons with MS (<6.5 on the Expanded Disability Status Scale [EDSS]) and found that performance was associated with activities of daily living, baseline HR, and subjective symptomatic fatigue in ambulatory patients but that there was no association with level of neurological impairment, respiratory muscle weakness, or lung function.24
The number of comorbidities increases with age, and this influences both walking ability and functional dependence.7,30 The clinical course of MS and the rate of disability progression are known to be related to age.31 Existing studies on 6MWT performance in persons with MS have not addressed the impact of ageing with MS and the potential for early decline in community function and independent living. It may be that individuals with worsening disease present with lower 6MWT scores at an earlier age, which would affect their ability to retain community-level function.
The purpose of this study was to determine the extent to which health factors, functional measures, and pulmonary impairment explain performance on the 6MWT in persons with MS. Explanatory factors considered for entry into the regression model in this study included subject characteristics (age, gender, race, height, weight, BMI); health factors (EDSS, number of medications, number of comorbidities, resting HR, systolic and diastolic blood pressure [BP]); physical performance (functional stair test [FST], sit-to-stand test [SST], static standing balance [BAL], Fatigue Severity Scale [FSS], Activities-specific Balance Confidence [ABC] Scale); and pulmonary function (observed and predicted FEV1, forced vital capacity [FVC], maximal mandatory ventilation [MVV], maximal expiratory pressure [MEP], maximal inspiratory pressure [MIP]). Secondary purposes of this study were (1) to compare 6MWT performance in people with MS who have mild and moderate disability and (2) to examine the effects of age on explanatory factors for 6MWT performance.
METHODS
Participants
A total of 64 ambulatory individuals (23 male, 41 female) with clinically diagnosed MS from two locations participated in this study. Individuals with clinically diagnosed MS who were at least 18 years of age and ambulatory (with or without assistive devices) were recruited for the study. Potential participants were excluded if they had an acute respiratory infection diagnosed by a physician, oral temperature >100°F (37.8°C), or an unstable cardiopulmonary or musculoskeletal condition unrelated to MS that could affect performance or safety.32 Individuals were also excluded if they scored >6.5 on the EDSS, a score that indicates that a person is no longer able to ambulate beyond 5 m without physical assistance.33 Current smokers were also excluded from participation. Participants were recruited through a television interview on a local news station as well as through personal solicitation at meetings and events of local MS support groups. Although 70 participants were recruited, 6 did not meet inclusion criteria after a review of their baseline vitals and health history. This study was conducted at two sites and was approved by the Institutional Review Boards of the University of Michigan—Flint (Site 1) and Duquesne University (Site 2). Informed consent was obtained from all participants.
The 6-Minute Walk Test
The 6MWT test was conducted according to American Thoracic Society standards, using procedures established by Fry and Pfalzer.13,34 The 6MWT test has high reliability (ICC: 0.95–0.99) in persons with MS34 and is responsive to changes in deteriorating status in persons with MS.29 A single repetition of the 6MWT was deemed appropriate, both in order to avoid participant fatigue and because a single repetition of the 6MWT was previously determined to be reliable in the MS population.34 Subjects were instructed to walk as quickly and safely as possible for 6 minutes. At Site 1, the course was a 91.5 m hallway with two turns; at Site 2, the course was located in a room 120 m long, where participants walked in a path that required two turns. There was no specific warm-up activity for participants. Each participant received two or three encouraging comments during the test. Participants were allowed to use an assistive device and to rest when necessary; a researcher walked to one side and behind the participant, close enough for safety purposes. The distance was recorded in feet and converted to metres. Distance walked on the 6MWT was compared against data from healthy community-dwelling adults (using age, gender, height, and weight factors in the calculation) to determine a difference score,19 which represents a functional walking deficit in the study participants with MS relative to healthy community-dwelling adults.
Health Factors
Factors related to health condition were obtained from a health intake questionnaire (HIQ) and included (1) physician classification of the participant's MS (i.e., relapsing–remitting, secondary progressive, primary progressive, or progressive relapsing), along with onset date and duration of MS; (2) prescription and over-the-counter medications; (3) prior surgeries; (4) any known medical conditions; and (5) any symptoms from a list provided, adapted from the conceptual model “Life Threat,” which elicits data on conditions noted for affecting morbidity and mortality. Each condition is assigned a score of 0, 1, or 2 based on presence and severity of the condition, and scores for individual conditions are summed for a total score.35 The Life Threat model is effective in discriminating changes in severity of comorbidity associated with ageing.36
Resting measures of HR, BP, respiratory rate, oxygen saturation, oral body temperature, height (inches), and weight (lb) were recorded. BMI was calculated by converting inches to metres and pounds to kilograms, then dividing weight by height (kg/m2). Level of disability was determined using the EDSS, based on a neurological exam (for pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, and cerebral functions) and ambulatory status for each subject.33 Investigators were trained by a health professional experienced in performing the EDSS in persons with MS. Mild disability was defined as an EDSS score <4.0 and moderate disability as an EDSS score of 4.0–6.5.
Subjects self-reported their fatigue and balance confidence using the FSS and ABC Scale respectively. The FSS is a nine-item questionnaire designed to measure severity of fatigue in activities of daily living;37 higher scores indicate greater levels of fatigue. The FSS has high internal consistency, test–retest reliability (r=0.84), and sensitivity in the MS population.37 The FSS was administered during the intake session. The ABC Scale is a 16-item questionnaire rating level of confidence in performing situation-specific activities such as “picking up a slipper from the floor”; each item is scored from 0% (no confidence) and 100% (full confidence in the ability to perform the activity without losing balance or becoming unsteady). The ABC Scale is strongly associated with measures of balance (timed up-and-go and Berg Balance Scale) in community-dwelling elderly and has good test–retest reliability (r=0.92).38,39 The ABC Scale was administered during the intake session at Site 2 and included as a post-study measure at Site 1.
Physical Performance Measures
Functional abilities of participants were examined using tests of static standing balance (BAL), a timed functional stair test (FST), a timed multiple sit-to-stand test (SST), and the 6MWT. All physical performance measures met the criteria for outcome measures established by the American National Multiple Sclerosis Society and have good test–retest reliability (ICC: 0.81–0.98) in persons with mild to moderate disability from MS.34,40
The BAL, FST, and SST were all administered using procedures established by Fry and Pfalzer.34 The best of three trials was used for data analysis for each measure. Safety precautions during the physical tests included gait belts worn by all subjects for all tests, guarding (as needed), and manual assistance if a fall was imminent. BAL was tested using single-limb stance (or tandem stance for those unable to maintain single-limb stance for at least 3 seconds) for up to 30 seconds. BAL time results were scored as modified from Guralnik et al., as follows11:
0.5=0–9 s tandem
1.0=10–19 s tandem
1.5=20–29 s tandem
2.0=30 s tandem
2.5=3–9 s single limb
3.0=10–19 s single limb
3.5=20–29 s single limb
4.0=30 s single limb
In the FST, patients were asked to ascend four steps, turn, and descend four steps, using a handrail as necessary. Hernandez et al. found that strength in ankle dorsiflexors, ankle plantarflexors, and knee extensors was significantly lower in community-dwelling ambulatory persons who reported difficulty with stooping, kneeling, and crouching tasks.41 The FST uses the same muscles measured in the study by Hernandez et al., which suggests that it is a viable test of functional lower-extremity strength and power.41 The FST, as a timed test, is a primary measure of lower-extremity power (i.e., a measure of work per unit time). Power was calculated using the following equation: (number of steps * step height [m] * body weight)÷(FST time [sec])=kg-m/min).32 Direct measures of muscle power can be provided by isokinetic devices, which measure movement with variable resistance and constant velocity. In a study involving people post stroke, muscle power was linked to gait speed.42 Kim and Eng demonstrated moderate correlations between lower-extremity isokinetic muscle power and both gait and stair-climbing velocity in individuals post stroke.42
In the SST, subjects were instructed to rise from a chair to a full standing position and return to sitting six times; time to complete the task was measured. The SST exhibits moderately high correlations with one-repetition maximum isotonic leg-press strength in older adults and is thus used as a functional test of strength.43 In patients who may not be able to complete a 6MWT (e.g., patients in acute care or home health care settings or those with limitations due to MS fatigue), the FST and SST are clinically useful substitute tests of functional mobility.
Pulmonary Function Measures
Each participant was tested for FVC, FEV1, MEP, MIP, and MVV. At Site 1, pulmonary function tests were performed in the following order for each test session: flow volume loop (FVC, FEV1), maximal inspiration and expiration testing (MIP, MEP), and MVV testing. The testing order was randomized at Site 2. The equipment used for determining pulmonary function differed between the two sites. At each site, stringent methods were employed to ensure reliability and validity of pulmonary function tests. Both sites calibrated their equipment according to the American Thoracic Society and European Respiratory Society Statement on Respiratory Muscle Testing and the manufacturers' recommendations.44 At Site 1, all pulmonary function testing was performed on the Vmax metabolic cart (Sensor Medics Corp, Yorba Linda, CA); Vmax pulmonary test software applied routine measures of consistency and accuracy imbedded in the test protocol. At Site 2, testing was performed using hand-held digital devices typically used in clinical settings: a micro spirometer (#051-06) for testing FVC and FEV1 and a respiratory pressure meter (#RPM01) with small controlled leak at the mouthpiece for testing MEP and MIP (Micro Direct, Lewiston, ME), and a portable spirometer (Renaissance PB 100) for testing MVV (Puritan Bennett, Boulder, CO). All devices were FDA approved and reported ATS certification for validity limits.45 The respiratory pressure meters use piezoelectric transducers (±3% accuracy, 1 cm resolution), which are now considered an acceptable method of measuring respiratory muscle strength.44
At both sites the test position was standardized: the participant assumed a sitting position in a chair with a backrest, with arms resting unsupported in the lap. Spoken instructions were provided following an instructor demonstration. Nose clips were used for all test manoeuvres. For MIP testing, the participant exhaled to residual volume, placed the mouthpiece in his or her mouth, and inspired maximally. For MEP testing, the participant inhaled maximally to total lung capacity, placed the mouthpiece in his or her mouth, and then blew the air out as hard and as fast as possible. The MIP and MEP manoeuvres were sustained for a minimum of 1.5 seconds. Each test was performed a minimum of two times and until values were within 10% of each other. The best trial was accepted and used for data analysis. Predicted values were calculated using normative values and prediction equations published in the literature (Knudson et al. for FEV1/FVC,46 Cherniak and Rabner for MVV,47 and Black and Hyatt for MIP/MEP48).
Procedures
To ensure consistency in test methods and administration between the two sites, a training film and written procedures were developed, and all assessors were trained using these tools. Subjects first completed the initial screening, HIQ, FSS, and ABC questionnaires. Pulmonary function tests were then administered. Upon completion of the pulmonary function tests, physical performance tests were administered in the following order for each participant: BAL, FST, SST, and 6MWT. Participants were given rest periods of 1–5 minutes between tests and between trials as needed.
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS), version 14.0 (SPSS Inc., Chicago, IL), was used for data analysis. Descriptive statistics (mean, SD, minimum, maximum) were calculated for the entire group and for participants with mild (EDSS<4.0) and moderate (EDSS 4.0–6.5) disability levels. Descriptive statistics were calculated for participant characteristics, health factors, physical performance, and pulmonary function (including corresponding percent predicted values). Frequency counts and percentages were calculated for gender and type of MS.
A one-way analysis of variance (ANOVA) was used to test for significant differences between the two levels of disability (mild and moderate) for various independent variables, including health factors, physical performance, and pulmonary function. The significance was set at α level p≤0.05.49 All variables were consistent with a normal distribution (Kolomogorov–Smirnov test) and homogeneity of variance (Levene's test). Because of the small sample size, we were concerned about the potential for a type II error resulting in failure to detect an important clinical difference, rather than a type I error resulting in failure to reject the null hypothesis. Therefore, a Bonferroni correction was not performed, to ensure that each variable was assessed for its own clinical importance.50 BAL was considered as interval data in the regression analysis. Before the regression was conducted, assumptions for using linear regression were tested and met (normal distribution, outliers, and linearity).51 Pearson product–moment correlations and partial correlations controlling for age were computed between the 6MWT and health factors, physical performance, and pulmonary function, with tests of significance to determine that r was not equal to zero. According to Portney and Watkins, correlation coefficients <0.25 demonstrate little to no association for these factors with the 6MWT; correlation coefficients between 0.25 and 0.49 indicate low association, 0.50–0.75 indicate moderate association, and coefficients >0.75 indicate a high association.51 For a factor showing a high correlation of 0.75, R2=0.56, meaning that this factor would explain 56% of the variance in 6MWT performance.51
Regression analyses used to generate the model for 6MWT (dependent variable) were conducted with the threshold for significance set at p<0.01 for inclusion and exclusion of independent variables that were significantly correlated with 6MWT and were considered clinically important contributors to ambulation, such as balance (BAL), balance confidence (ABC), and lower-extremity power (FST) and strength (SST). Since lower-extremity strength (SST) and power (FST) are related factors, a three-factor regression model including both of these factors is not valid. Balance confidence (ABC) and static balance (BAL), while similar, are not as strongly associated. The regression models to explain the variance in 6MWT were created using standardized β weights indicating the relative importance of each factor. A two-factor model and two simple (single-factor) regression models were explored to determine whether these models would explain as high a percentage of the variance in the 6MWT distance as the three-factor model. The two-factor regression and the two different simple regressions were modelled for 6MWT using the ABC and FST as their partial correlation; β weights were high, indicating a large, independent contribution to the 6MWT. The ABC was best modelled with a linear regression (see Figure 1). The FST appears curvilinear; it was modelled with both a linear regression and a non-linear regression, and the two models were compared (see Figures 2 and 3). The FST was best modelled as a non-linear polynomial regression with a quadratic of β2>0 (see Figure 3). As FST time increases on the x-axis, performance is poorer and tends to plateau, with consistently shorter distance walked on the 6MWT.
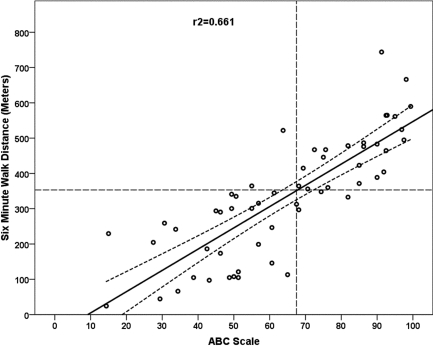
Figure 1.
Simple linear regression with 95% confidence interval bands for 6-minute walk distance (m) from Activities-specific Balance Confidence (ABC) Scale total score. The dashed intersecting lines indicate the distance older adult community ambulators can walk in 6 minutes and the intersection with ABC score on the x-axis.
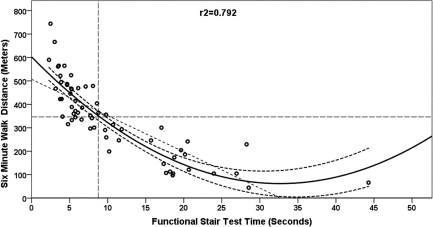
Figure 2.
Curvilinear (quadratic) regression with 95% confidence interval bands for 6-minute walk distance (m) from functional stair test (FST) time (seconds). The dashed intersecting lines indicate the distance older adult community ambulators can walk in 6 minutes and the intersection with FST time on the x-axis.
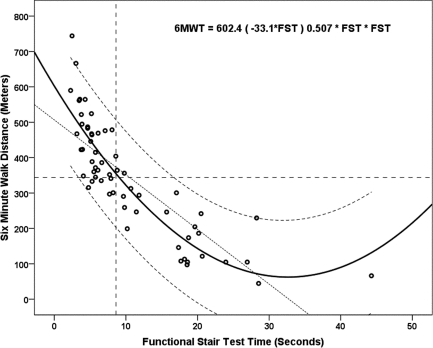
Figure 3.
Curvilinear (quadratic) regression with 95% individual prediction bands for 6-minute walk distance (m) from functional stair test (FST) time (seconds). The dashed intersecting lines indicate the distance older adult community ambulators can walk in 6 minutes and the intersection with FST time on the x-axis.
RESULTS
Between-Group Differences in Health Factors, Physical Performance, and Pulmonary Function
Participant characteristics and health factors are reported in Tables 1 and 2; data on physical performance measures and pulmonary function tests are reported in Table 3. Compared to participants with mild disability, participants with moderate disability were significantly older (p<0.001); expressed less balance confidence (ABC) (p<0.001); exhibited lower performance levels on all physical performance measures (6MWT, FST, SST, BAL) (p<0.001); and had lower observed MVV (p=0.002), lower predicted MIP (p=0.027), lower predicted MEP (p=0.047), and lower predicted MVV (p=0.003).
Table 1.
Participant Characteristics: Gender and Type of Multiple Sclerosis
| Characteristic | MS Total Group (n=64) |
Mild Disability EDSS<4.0 (n=43) |
Moderate Disability EDSS 4.0 to 6.5 (n=21) |
p* |
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gender |
0.220 |
||||||
| Female | 53 | 83 | 37 | 86 | 16 | 76 | |
| Male |
11 |
17 |
6 |
14 |
5 |
24 |
|
| Type of MS |
0.012 |
||||||
| Relapsing–remitting | 38 | 64 | 31 | 82 | 7 | 33 | |
| Secondary progressive | 11 | 19 | 3 | 8 | 8 | 38 | |
| Primary progressive | 6 | 10 | 2 | 5 | 4 | 19 | |
| Progressive relapsing | 4 | 7 | 2 | 5 | 2 | 10 | |
Chi-square test significant at p<0.05
MS=multiple sclerosis; EDSS=Expanded Disability Status Scale
Table 2.
Participant Characteristics: Health Factors
| MS Total Group EDSS ≤6.5 (n=64) |
Mild Disability EDSS <4.0 (n=43) |
Moderate Disability EDSS 4.0–6.5 (n=21) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Range | Mean | SD | 95% CI | Mean | SD | 95% CI | p |
| Age (years) | 49.3 | 9.8 | 23–77 | 46.6 | 9.2 | 43.5–49.3 | 54.9 | 8.8 | 50.7–58.7 | <0.001 |
| Male (n=23) | 51.0 | 10.7 | 28–77 | 46.0 | 9.1 | 53.8 | 8.1 | |||
| Female (n=41) | 48.2 | 9.4 | 23–69 | 47.5 | 10.0 | 55.5 | 10.3 | |||
| EDSS score | 3.8 | 1.6 | 1.5–6.5 | 2.8 | 0.5 | 2.6–2.9 | 6.1 | 0.74 | 5.5–6.3 | <0.001 |
| Number of comorbidities | 2.9 | 3.0 | 0–7 | 2.7 | 2.2 | 1.7–3.0 | 3.3 | 4.1 | 1.2–3.3 | 0.441 |
| Number of medications | 5.2 | 3.3 | 1–15 | 4.8 | 2.8 | 4.0–5.8 | 6.0 | 4.2 | 3.9–7.8 | |
| Fatigue (FSS) | 5.1 | 1.3 | 1.3–7.0 | 5.2 | 1.2 | 4.8–5.6 | 5.1 | 1.5 | 4.4–5.7 | 0.999 |
| Weight (kg) | 80.1 | 19.5 | 47.2–134.3 | 81.2 | 21.0 | 74.6–88.1 | 77.7 | 16.2 | 69.7–84.4 | 0.518 |
| Male | 83.8 | 20.1 | 56.7–123.6 | 85.5 | 22.4 | 81.5 | 17.6 | |||
| Female | 77.7 | 19.6 | 47.2–134.3 | 79.5 | 21.2 | 73.1 | 14.3 | |||
| Height (m) | 1.67 | 0.10 | 1.42–1.89 | 1.67 | 0.10 | 1.64–1.70 | 1.68 | 0.10 | 1.63–1.72 | 0.524 |
| Male | 1.71 | 0.10 | 1.52–1.88 | 1.75 | 0.09 | 1.69 | 0.10 | |||
| Female | 1.65 | 0.10 | 1.42–1.89 | 1.64 | 0.07 | 1.66 | 0.10 | |||
| BMI (kg/m2) | 28.3 | 6.2 | 18.2–45.0 | 29.0 | 6.7 | 26.8–31.2 | 27.3 | 4.5 | 25.4–29.5 | 0.294 |
| HR (bpm) | 78.2 | 10.4 | 55–108 | 79.0 | 11.4 | 75.3–82.6 | 76.6 | 8.0 | 73.3–80.1 | 0.556 |
| Systolic BP (mm Hg) | 125.8 | 17.7 | 100–178 | 126.1 | 18.7 | 120.9–132.8 | 125.7 | 16.5 | 115.6–130.3 | 0.941 |
| Diastolic BP (mm Hg) | 81.8 | 8.8 | 62–100 | 81.9 | 9.6 | 79.4–85.3 | 80.7 | 6.1 | 76.7–83.5 | 0.599 |
| ABC Score** | 64.4 | 22.5 | 14.4–99.4 | 74.6 | 18.2 | 69.2–81.5 | 45.1 | 16.5 | 38.4–54.1 | <0.001 |
| Male | 63.6 | 21.8 | 14.4–98.1 | 79.2 | 19.3 | 67.5–90.9 | 49.2 | 18.8 | 35.7–62.7 | |
| Female | 66.1 | 24.1 | 15.0–99.4 | 73.3 | 17.3 | 65.7–80.7 | 43.6 | 16.1 | 32.8–54.4 | |
One-way ANOVA significant at p<0.05
n=64 (except n=58 for ABC scores)
MS=multiple sclerosis; CI=confidence interval; EDSS=Expanded Disability Status Scale; FSS=Fatigue Severity Scale
BMI=body mass index; HR=heart rate; bpm=beats per minute; BP=blood pressure; ABC=Activities-specific Balance Confidence Scale
Table 3.
Physical Performance Measures and Pulmonary Function Tests for Ambulatory Participants with Multiple Sclerosis
| MS Total Group EDSS ≤6.5 (n=64) |
Mild Disability EDSS <4.0 (n=43) |
Moderate Disability EDSS 4.0–6.5 (n=21) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | 95% CI | Mean | SD | 95% CI | p* |
| Physical Performance | |||||||||
| 6MWT (m) | 337.2 | 160.7 | 402.4 | 136.7 | 361.5–449.1 | 193.7 | 108.5 | 153.3–255.8 | <0.001 |
| Male | 362.8 | 169.2 | 459.5 | 133.8 | 237.0 | 122.4 | |||
| Female | 322.2 | 156.4 | 380.1 | 156.0 | 175.0 | 99.2 | |||
| 6MWT Pred (m)** | 531.3 | 54.5 | 543.0 | 51.4 | 526.7–559.2 | 508.4 | 54.1 | 483.7–533.0 | |
| % of predicted distance walked*** | 62.8 | 27.8 | 74.3 | 23.2 | 67.0–81.6 | 40.2 | 21.8 | 30.3–50.1 | |
| Male | 66.6 | 27.1 | 81.6 | 19.4 | 47.2 | 23.8 | |||
| Female | 60.5 | 28.3 | 71.0 | 24.4 | 33.8 | 18.5 | |||
| FST (s) | 10.7 | 8.4 | 7.5 | 4.4 | 5.9–8.8 | 17.9 | 10.2 | 12.4–22.0 | <0.001 |
| FST power (kg-m/min) | 438.7 | 302.7 | 532.7 | 302.0 | 437.4–628.0 | 246.0 | 198.1 | 153.3–338.7 | <0.001 |
| Male | 544.3 | 376.5 | 715.5 | 376.0 | 321.7 | 246.1 | |||
| Female | 374.8 | 230.7 | 447.9 | 220.7 | 170.3 | 97.5 | |||
| SST (s) | 22.5 | 15.7 | 18.0 | 5.9 | 16.1–20.0 | 32.5 | 23.4 | 20.3–41.3 | <0.001 |
| BAL (s) | 15.7 | 10.5 | 18.9 | 9.9 | 9.3 | 8.4 | |||
| BAL (category) |
2.8 |
.2 |
2.4–3.1 |
2.0 |
.3 |
1.4–2.6 |
<0.001 |
||
| Pulmonary Function Measures | |||||||||
| FEV1 (l) | 2.73 | 0.69 | 2.83 | 0.63 | 2.66–3.06 | 2.56 | 0.78 | 2.15–3.86 | 0.142 |
| FEV1 Pred (%) | 97.27 | 18.69 | 99.60 | 15.10 | 95.5–104.5 | 93.43 | 24.77 | 79.1–101.5 | 0.226 |
| FVC (l) | 3.69 | 0.91 | 3.68 | 0.88 | 3.45–4.00 | 3.53 | 0.97 | 2.96–3.87 | 0.534 |
| FVC Pred (%) | 99.80 | 16.48 | 100.47 | 15.54 | 96.0–105.8 | 98.35 | 18.82 | 86.5–104.5 | 0.740 |
| FEV1/FVC | 0.77 | 0.01 | 0.78 | 0.08 | 0.75–0.80 | 0.74 | 0.13 | 0.69–0.81 | 0.122 |
| MIP (cm H2O) | 62.1 | 27.3 | 65.2 | 27.0 | 57.9–75.1 | 55.9 | 27.6 | 40.5–64.3 | 0.207 |
| MIP Pred (%) | 64.4 | 30.0 | 70.2 | 29.8 | 60.3–78.4 | 52.6 | 27.5 | 45.0–69.6 | 0.027 |
| MEP (cm H2O) | 79.1 | 37.2 | 84.5 | 37.9 | 73.6–98.1 | 67.9 | 33.7 | 50.7–71.4 | 0.095 |
| MEP Pred (%) | 49.3 | 20.8 | 52.9 | 19.3 | 45.2–59.8 | 41.9 | 21.9 | 28.1–50.3 | 0.047 |
| MVV (l/min) | 102.1 | 28.3 | 109.6 | 27.7 | 101.8–119.3 | 86.7 | 23.4 | 76.6–98.5 | 0.002 |
| MVV Pred (%) | 93.6 | 20.9 | 98.9 | 19.7 | 93.9–106.3 | 82.6 | 19.1 | 76–6-95.2 | 0.003 |
One-way ANOVA significant at p<0.05
Predicted 6MWT distance=493+(2.2×height [cm])−(0.93×weight [kg]) – (5.3×age). For men add 17 m. Subtract 100 m for lower limit of normal range.20
Percentage of predicted distance walked=(actual 6MWT÷predicted 6MWT)×100%.
MS=multiple sclerosis; EDSS=Expanded Disability Status Scale; 6MWT=6-Minute Walk Test distance; FST=Functional Stair Test; SST=Sit-to-Stand Test; BAL=single or tandem static standing balance; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; FEV1/FVC=forced expiratory volume in 1 second / forced vital capacity; MIP=maximal inspiratory pressure; MEP=maximal expiratory pressure; MVV=maximal voluntary ventilation
6MWT Correlations with Health Factors, Physical Performance, and Pulmonary Function
The associations between 6MWT and health factors, physical performance, and pulmonary function measures are shown in Table 4. Pearson product–moment correlations showed that FST, ABC, and BAL were all highly correlated with 6MWT distance in ambulatory persons with MS. After controlling for age, significant associations remained between the 6MWT and ABC scores (R2=0.594), EDSS scores (R2=0.346), physical performance (FST (R2=0.572), SST (R2=0.326), BAL (R2=0.156)), observed and percent predicted MIP (R2=0.079, R2=0.099), MEP (R2=0.163, R2=0.091), and MVV (R2=0.092, R2=0.103). The 6MWT was highly correlated with the ABC and FST, moderately correlated with the SST and EDSS, and showed a low correlation with BAL and with predicted and observed MIP, MEP, and MVV.
Table 4.
Health Factors, Physical Performance, and Pulmonary Function Correlated with 6-Minute Walk Test
| Measures Correlated with 6MWT |
Partial* |
R2 |
|---|---|---|
| Health Factors | ||
| EDSS | −0.588*** | 0.346 |
| Number of comorbidities | 0.026 | 0.001 |
| Number of medications | −0.056 | −0.001 |
| Fatigue (FSS) | 0.003 | 0.001 |
| BMI (kg/m2) | −0.065 | 0.004 |
| HR | −0.126 | 0.016 |
| Systolic BP | −0.180 | 0.032 |
| Diastolic BP | −0.200 | 0.040 |
| ABC score) |
0.771*** |
0.594 |
| Physical performance measures |
|
|
| FST | −0.756*** | 0.572 |
| SST | −0.571*** | 0.326 |
| BAL | 0.395*** | 0.156 |
| Pulmonary function measures |
|
|
| MIP observed | 0.281** | 0.079 |
| MIP predicted | 0.314** | 0.099 |
| MEP observed | 0.404*** | 0.163 |
| MEP predicted | 0.302** | 0.091 |
| MVV observed | 0.304** | 0.092 |
| MVV predicted | 0.321*** | 0.103 |
| FEV1 observed | 0.111 | 0.012 |
| FEV1 predicted | 0.113 | 0.013 |
| FVC observed | 0.043 | 0.002 |
| FVC predicted | 0.073 | 0.005 |
| FEV1/FVC | 0.086 | 0.007 |
Partial correlation controlling for age
p<0.05
p<0.01
6MWT=6-Minute Walk Test; EDSS=Expanded Disability Status Scale; BMI= body mass index; FSS=Fatigue Severity Scale; ABC=Activities-specific Balance Scale; FST=Functional Stair Test; SST=Sit-to-Stand Test; BAL=single-limb or tandem balance; HR=heart rate; BP=blood pressure; MIP=maximal inspiratory pressure; MEP=maximal expiratory pressure; MVV=maximal voluntary ventilation; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; FEV1/FVC=forced expiratory volume in 1 second / forced vital capacity
Step-wise Multiple Regression and Simple Regression Models
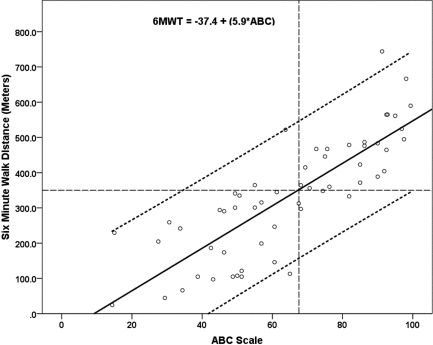
The 6MWT step-wise multiple regression model included ABC scores, FST, and BAL as significant independent explanatory factors in a three-factor model (ABC, FST, BAL), a two-factor model (ABC, FST), and single-factor models (ABC, Figure 1; FST, Figure 2), as shown in Table 5. The R2 values (Table 5) indicated that the ABC score explained 66% of the variance (see Figure 1); the regression equation was 6MWT distance=−37.4+(5.9×ABC). The FST linear regression model explained 65% of the variance; the regression equation was 6MWT distance=501.2+(−15.00×FST). When the linear model was adjusted to a curvilinear model, 79% of the variance in 6MWT performance was explained (see Figure 2). When ABC and FST variables were both included in a two-factor linear model, 75% of the variance was explained; the regression equation was 6MWT distance=218.5+((3.35×ABC score)−(9.09×FST time)). The three-factor linear model including ABC, FST, and BAL explained 77% of variance in 6MWT performance; the regression equation was 6MWT distance=207.9+((2.74×ABC score)−(9.07×FST time)+(3.08×single-limb BAL time)). The model that best explained 6MWT performance was the single-factor curvilinear model using the FST time (see Figure 3). Table 5 shows standardized coefficients (β weights).
Table 5.
Predictor Variables of Six-Minute Walk Distance and their Unstandardized Coefficients and Standardized Coefficients (β) and Significance
| Predictor Variables | Unstandardized Coefficients* | Standardized Coefficients (β) | R2 | 95% CI | p |
|---|---|---|---|---|---|
| Three-factor linear regression model (n=58) |
|
|
|
|
|
| Constant | 207.9 | ||||
| ABC Score | 2.7 | 0.434 | 0.768 | 1.6–45.0 | <0.001** |
| FST time (s) | −9.1 | −0.433 | −12.1–−4.5 | <0.001** | |
| BAL time (s) |
3.1 |
0.147 |
|
0.040–4.4 |
0.046*** |
| Two-factor linear regression model (n=58) |
|
|
|
|
|
| Constant | 218.5 | ||||
| ABC Score | 3.4 | 0.485 | 0.750 | 1.97–4.89 | <0.001** |
| FST time (s) |
−9.1 |
−0.441 |
|
−12.40–−4.5 |
|
| Single-factor regression models | |
|
|
|
|
| Constant | −37.4 | ||||
| ABC Score (n=58) (linear) | 5.9 | 0.815 | 0.664 | 4.7–6.7 | <0.001** |
| Constant | 501.2 | ||||
| FST time (s) (n=64) (linear) | −15.0 | −0.804 | 0.661 | 460.6–539.6 | <0.001** |
| Constant | 602.4 | ||||
| FST time (s) (n=64) (curvilinear/quadratic) | β 1=−33.1 | β 2=0.507 | 0.792 | <0.001** |
Thresholds levels for 6MWT were set at 350 m and marked on each plot (see Figures 3 and 4) for each of the main explanatory variables. The threshold level of 350 m was chosen based on Enright et al.'s report of a mean 6MWT distance of 344 m (SD=88 m) in 2,281 community-dwelling adults over age 68.20 Previous studies found that 350 m was the minimum distance required for community functioning, and distances less than 350 m were associated with a substantial increase in risk of mortality.25,26,52–54 Figure 4 depicts the regression of ABC scores against 6MWT distance, where an ABC score of 65 corresponds to a distance of 350 m on the 6MWT, indicating that participants scoring at or below 65 on the ABC Scale are at risk for low 6MWT scores. Figure 3 depicts the regression of FST against 6MWT distance, where an FST of 8 s corresponds to a distance of 350 m on the 6MWT, indicating that participants who required 8 seconds or more to complete the FST were likely to have low 6MWT scores.
Figure 4.
Simple linear regression with 95% individual prediction bands for 6-minute walk distance (m) from Activities-specific Balance Confidence (ABC) total score. The dashed intersecting lines indicate the distance older adult community ambulators can walk in 6 minutes and the intersection with ABC score on the x-axis.
Influence of Age on 6MWT and ABC Scores
Table 6 displays results of the 6MWT, ABC, and FST according to age categories (5-year periods) for the entire group of participants. These results indicate that individuals with MS who are younger (<50 years of age) are likely to be above the thresholds for 6MWT, balance confidence, and stair-climbing performance.
Table 6.
6-Minute Walk Distance, Balance Confidence Score, and Functional Stair Test Time by Age Category
| 6-Minute Walk Distance (m) Pearson product–moment correlation for 6MWT with age (r=−0.464**) | |||||
|---|---|---|---|---|---|
| MS Subjects |
Reported Data for Healthy Adults |
||||
| Age Category | n | % | Mean | SD | Mean±SD |
| <40 | 10 | 15.6 | 472.3 | 143.1 | 614±69*,24 577±56*,28 593±57***,15 638±44****,15 |
| 40 to <45 | 8 | 12.5 | 385.5 | 124.8 | 670±85***,22 671±56****,22 |
| 45 to <50 | 10 | 15.6 | 368.3 | 180.1 | |
| 50 to <55 | 18 | 28.1 | 276.2 | 138.2 | |
| 55 to <60 | 12 | 18.8 | 308.0 | 140.4 | 494***,19 576****,19 |
| ≥60 |
6 |
9.4 |
206.1 |
122.1 |
530±77***,14 458±136****,14 659±62*,16 631±57***,16 690±53****,16 344±8820 631±93*,21 589***,21 673****,21 |
|
ABC Scores Pearson product–moment correlation for ABC with age (r=−0.416**) | |||||
|
MS Subjects |
|||||
|
Age Category |
n |
% |
Mean |
SD |
|
| <40 | 9 | 15.5 | 78.7 | 21.7 | |
| 40 to <45 | 8 | 13.8 | 75.4 | 16.6 | |
| 45 to <50 | 8 | 13.8 | 69.1 | 24.0 | |
| 50 to <55 | 16 | 27.6 | 55.1 | 19.5 | |
| 55 to <60 | 11 | 19.0 | 64.9 | 25.9 | |
| ≥60 |
6 |
10.3 |
46.0 |
7.0 |
|
|
FST Time (s) Pearson product–moment correlations for FST with age (r=0.425**) | |||||
|
MS Subjects |
|||||
|
Age Category |
n |
% |
Mean |
SD |
|
| <40 | 10 | 15.6 | 5.7 | 5.0 | |
| 40 to <45 | 8 | 12.5 | 7.9 | 4.4 | |
| 45 to <50 | 10 | 15.6 | 10.3 | 7.7 | |
| 50 to <55 | 18 | 28.1 | 12.9 | 8.4 | |
| 55 to <60 | 11 | 18.8 | 9.4 | 5.4 | |
| ≥60 | 6 | 9.4 | 20.2 | 13.7 | |
mean 6MWT includes both sexes
significant at p=0.01
mean 6MWT for female participants
mean 6MWT for male participants
6MWT=6-Minute Walk Test; MS=multiple sclerosis; ABC=Activities-specific Balance Confidence Scale; FST=Functional Stair Test
Data sources: Savci et al.24 (n=30; 17♀/13♂) in MS study (mean age: 35±9 yrs); Chetta et al.28 (n=10; 7♀/3♂) in MS study (mean age: 33±8 yrs); Chetta15 (mean age ♀=33±9 yrs; mean age ♂=36±8 yrs); Gibbons22; Enright and Sherrill19; Enright et al.20 (n=173; mean age ♀=62 yrs; mean age ♂=59.5); Camarri et al.16 (n=37; ♀; mean age=64.5±5.2 yrs); Troosters et al.21 (n=54; mean age=65±10 yrs); Lord et al.14 (n=24 ♀; n=5 ♂; age: min=62, max=69 yrs)
DISCUSSION
These results indicate that measures of balance confidence (ABC), static standing balance (BAL), and lower-extremity power (FST) are highly associated with and explain a large portion of the variance in performance on the 6MWT in persons with MS. Of these, balance confidence and lower-extremity power explained the most variance in 6MWT performance. This result is consistent with studies of non-disabled, community-dwelling older adults, which found that those with reduced balance confidence demonstrated decreased lower-extremity strength and walking speed.55,56 Likewise, several other studies of older community-dwelling individuals reported an association between 6MWT and measures of physical performance, activities of daily living, or self-reported activity.14,19,57
Savci et al. reported that functional independence in carrying out activities of daily living (Barthel Index) explained 61% of the variance in 6MWT performance in a similar group (EDSS<6.5) of individuals with MS.24 While they did not include other measures of physical performance or balance in their regression modelling, adding baseline HR and FSS to their model explained 81% of the variance in 6MWT distance (6% explained by the FSS).24 Neither the fatigue data from our study (FSS) nor those from Chetta et al., who used the Modified Fatigue Impact Scale, were highly correlated with 6MWT.28 Compared to participants in our study, participants in the Savci et al. study had higher resting HR (94.07±15.40 bpm vs. 78.2±10.4 bpm), despite being younger (34.97±8.19 years vs. 49.3±9.8 years).24 Similarly, Chetta, et al. reported higher resting HR in those with mild MS (EDSS <4.0) relative to our mild group (86±10 bpm vs. 79.0±11.4 bpm).28 Lower HRs for participants in our study may be related to the fact that smokers were excluded from our study but not from previous studies. It is also possible that the lower resting HR of our study participants reflected higher overall fitness levels relative to those in Savci et al.'s and Chetta et al.'s studies, resulting in a weaker association between fatigue and 6MWT performance in our study.24,28
Gait velocity has also been correlated with strength of the hamstring and quadriceps muscles in persons with MS.58 In persons with stroke, the FST has been demonstrated to be both a test of function and a test of lower-extremity muscle strength and power.42 The decreased 6MWT performance associated with decreased lower-extremity power, as demonstrated on the FST, is consistent with the findings of Hernandez et al., who found that lower-extremity muscle strength was significantly reduced in community ambulatory persons who reported difficulty with stooping, kneeling, and crouching tasks.41 As a single factor in a curvilinear regression model, the FST explained 79% of the variance of the 6MWT in our study, which suggests that lower-extremity strength and power contribute significantly to ambulatory endurance at functional distances associated with the 6MWT test in persons with MS (see Table 5).
Respiratory muscle performance measures (predicted MIP, MEP, and MVV) were significantly and positively associated with 6MWT performance in our study, indicating that better respiratory muscle function was associated with greater walking distance. MIP, MEP, and MVV each individually explained about 10% of the variance in 6MWT distance (see Table 4). While this may not appear to be highly important, when we consider the variance explained by BMI, number of medications, number of comorbidities, and fatigue—each of which individually explained less than 1% of the variance—the association of MIP, MEP, and MVV with the 6MWT seem likely to be clinically relevant.
Ventilatory muscle weakness, incoordination, and postural abnormalities are thought to reduce MEP, MIP, and MVV in persons with MS.59,60 This is true even in those individuals who are ambulatory.61,62 Average MIP and MEP values in our study were lower than those reported by Chetta et al. and by Savci et al., who found no association between measures of MIP or MEP and 6MWT performance.24,28 Participants in our study were, on average, 15 years older than those examined by Chetta et al. (32±7 vs. 46.6±9.2 years, mild) and Savci et al. (34.97±8.19 vs. 49.3±9.8 years, moderate). It is possible that respiratory muscle performance may be more important as the individual with MS ages, especially since MS disability is known to be related to age.31 Respiratory complications have been identified as a major cause of morbidity and mortality in individuals with advanced MS.5
We found that 6MWT performance differed significantly between participants with mild disability (EDSS <4.0, 6MWT mean=402.4 m) and those with moderate disability (EDSS 4.0–6.5, 6MWT mean=193.7 m). The difference in 6MWT distance was explained by the differences in measures of balance confidence (ABC) and lower-extremity power (FST Power, FST Time). The participants with moderate disability walked more slowly than those with mild disability (6MWT=193.7 m vs. 402.4 m; see Table 3) and consistently demonstrated decreased respiratory muscle function, as indicated by predicted MIP (52.6% vs. 70.2%), MEP (41.9% vs. 52.9%), and MVV (82.6% vs. 98.9%). Resisted breathing training has been shown to reverse or prevent further decline in ventilatory muscle function in persons with MS.62–65
Age also affected 6MWT distance (see Table 6). Of the 64 participants in this study, 28 were under age 50. Of these 28 participants, only 3 (10.7%) were moderately disabled; by contrast, of the 36 participants aged 50 or older, 18 (50.0%) were moderately disabled. Participants in this study exhibited reduced walking distances on the 6MWT relative to non-disabled individuals 20–50 years of age (593±57 m for women, 638±44 m for men15) and 60–69 years of age (530±77 m for women, 458±136 m for men14). Data on 6MWT distance for healthy individuals are included in Table 6.14,15,19–22,24,28 After age 70, the average 6MWT distance falls below 500 m but remains above the 350 m threshold required for community ambulation.14,20 The 6MWT distance in the moderately disabled group of individuals with MS were smaller than distances reported as necessary for even single-task community outings.66,67 Thus, individuals with MS who are over 50 years of age and moderately disabled are at risk of losing independent functional community ambulation.
Similar declines in function were observed with age in the ABC and FST (see Table 6). In this study, ABC scores generally declined with age and were very low for participants with moderate disability; the ABC is known to be related to balance ability during functionally based tasks in community-dwelling elderly people.38 On the FST, participants with moderate disability took twice as long as those with mild disability to complete the stair-climbing task. Stair-climbing ability has also been identified as an important factor for maintaining community functioning.8 Declines in balance confidence and stair-climbing ability observed both with increasing age and with increasing EDSS levels are thus additional indicators of risk of decreased community mobility in persons with MS.
In ambulatory persons with MS who have mild or moderate disability, the ABC and FST, when modelled together, explained 75% of the variance in 6MWT. When modelled in single-factor regressions, the ABC explained 66% and the FST explained 79% of the variance in 6MWT distance, which indicates that both ABC performance and FST performance are good explanatory factors of 6MWT performance. The two-factor regression model (ABC and FST) and both single-factor regression models explained 6MWT distance through most of the range of 6MWT scores, with some variance in the highly able walkers (see Figures 1 and 2). Individuals with MS who scored <65 on the ABC or required more than 8 seconds to complete the FST did not achieve the 350 m distance threshold on the 6MWT. Participants who were older exhibited higher levels of disability, reduced 6MWT distances, reduced balance confidence, and increased FST time.
Both the ABC and FST are readily available clinical measures that require limited time to administer. Use of the ABC and FST as indicators of 6MWT performance may be very helpful in clinic situations where there is inadequate space to conduct the 6MWT or in cases where the 6MWT would produce excessive fatigue in a patient with MS. If a single-factor regression model is used to explain the 6MWT distance, the FST is preferred because of its larger standardized coefficient (β) of −8.804. The FST simply requires the participant to complete three trials of ascending and descending four steps as quickly as possible and can typically be administered in no more than 3 minutes. For many participants this is less fatiguing than completing a 6MWT. If, for some reason, the patient is unable to climb stairs safely, then the ABC single-factor regression model is recommended. The ABC is a questionnaire that can be self-administered.
This is the first report describing an association between 6MWT and scores on the ABC scale in persons with MS. Hatch et al. reported that average ABC scores for older community-dwelling individuals at risk for falls ranged from 62 to 69.38 To determine ABC scores that would indicate risk for decreased community ambulation in persons with MS, we compared participants' performance to a minimum standard of 350 m for community ambulation.52,66,67 Using the 350 m standard for community ambulation in an older population,20 individuals with MS in this study whose ABC score fell below 65 would be expected to have decreased community ambulation (see Figure 1). This value is in agreement with those reported by Hatch et al.38 All moderately disabled participants in this study fell well below this threshold. ABC scores and 6MWT have been shown to relate to indices of satisfaction with community reintegration after stroke and may also be important measures for those with MS.68
A similar comparison of stair-climbing speed (FST) with a 350 m standard for the 6MWT showed that participants with an FST time >8 seconds demonstrated decreased community ambulation (see Figure 2). Almost all participants in the moderately disabled group required more than 8 seconds to complete the stair-climbing assessment and are thus likely to have limited mobility. However, those in the mildly disabled group did not appear to have limited mobility.
In healthy older adults, agility and resistance training have been shown to improve balance confidence.69 Since balance confidence and lower-extremity power are strongly linked with ability to walk farther on the 6MWT in persons with MS, studies of interventions to improve these factors in persons with MS are indicated. These studies should be designed with outcome measures that assess functional gait skills as well as participation in the community. The relationship between balance confidence and the need for assistance with daily living tasks is also an area of interest for future study.
LIMITATIONS
This study has several limitations. Use of two different study sites created a potential threat to the study's internal validity as a result of the use of two different measurement devices for pulmonary function tests and of different walking paths for the 6MWT. Another major limitation is that the ABC scale was not administered at a consistent time at the two sites (i.e., it was administered during the intake session at one site and included as a post-study measure at the other site). Some participants in our study expressed cautionary statements about overexertion and knowledge about pacing activities to avoid fatigue. We did not formally examine their knowledge of fatigue management, and individuals educated on principles of overuse may have paced themselves differently on the 6MWT test and been more sensitive to factors that contribute to fatigue. Also, since participants who currently smoke were excluded from this study, the functional test data reported here may reflect higher values than would be found in a MS population that includes smokers. Because of the lower incidence of MS in men and the relatively small sample size, with very few men (23 vs. 41 women), analysis by gender was not possible.
CONCLUSION
The ABC and FST are inexpensive, readily accessible tests that may be completed in just a few minutes in the clinic, and they are highly explanatory of 6MWT distance in persons with MS. Incorporating the ABC, FST, and 6MWT into routine primary medical or physical therapy examinations will provide valuable insight into the risk of loss of community ambulation ability in mildly and moderately disabled persons with MS.
KEY MESSAGES
What Is Already Known on This Topic
Several studies have reported an association between 6MWT distance and measures of physical performance, activities of daily living, or self-reported activity in older community-dwelling individuals.14,19,47 Low scores on the 6MWT explain early disability and loss of community functioning. 6MWT distance is significantly reduced in persons with MS, which suggests that they are at risk for mortality and impaired mobility.24,28,29
What This Study Adds
This study reports on clinical characteristics and physical performance measures related to 6MWT as alternative methods to explain risk of disablement. Performance on the 6MWT, FST, and ABC scale is influenced by age and disability in persons with MS. This study demonstrates that efficient clinical measures of stair climbing (FST) and balance confidence (ABC Scale) explain 75% of the variance in 6MWT performance. The FST and ABC scale may be practical clinical measures for identifying the potential for early loss of community function and independent living in persons with MS.
Wetzel JL, Fry DK, Pfalzer LA. Six-minute walk test for persons with mild or moderate disability from multiple sclerosis: performance and explanatory factors. Physiother Can. 2010;preprint. doi:10.3138/ptc.2009-62
REFERENCES
- 1.Haselkorn JK, Leer SE, Hall JA, Pate DJ. Mobility. In: Burks JS, Johnson KP, editors. Multiple sclerosis: diagnosis, medical management, and rehabilitation. New York: Demos; 2000. pp. 323–32. [Google Scholar]
- 2.Petajan JH. Weakness. In: Burks JS, Johnson KP, editors. Multiple sclerosis: diagnosis, medical management, and rehabilitation. New York: Demos; 2000. pp. 307–21. [Google Scholar]
- 3.Krupp LB, Elkins LE. Fatigue. In: Burks JS, Johnson KP, editors. Multiple sclerosis: diagnosis, medical management, and rehabilitation. New York: Demos; 2000. pp. 291–7. [Google Scholar]
- 4.Mutluay FK, Gurses HN, Saip S. Effects of multiple sclerosis on respiratory functions. Clin Rehabil. 2005;19:426–32. doi: 10.1191/0269215505cr782oa. doi: 10.1191/0269215505cr782oa. [DOI] [PubMed] [Google Scholar]
- 5.Redelings MD, McCoy L, Sorvillo F. Multiple sclerosis mortality and patterns of comorbidity in the United States from 1990 to 2001. Neuroepidemiology. 2006;26:102–7. doi: 10.1159/000090444. doi: 10.1159/000090444. [DOI] [PubMed] [Google Scholar]
- 6.Pittock SJ, Mayr WT, McClelland RL, Jorgensen BS, Weigand SD, Noseworthy JH, et al. Disability profile of MS did not change over 10 years in a population-based prevalence cohort. Neurology. 2004;62:601–6. doi: 10.1212/wnl.62.4.601. [DOI] [PubMed] [Google Scholar]
- 7.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence: unifying the approach to geriatric syndromes. J Am Med Assoc. 1995;273:1348–53. doi: 10.1001/jama.273.17.1348. [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penninx BWJH, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A-Biol. 2000;55:M691–7. doi: 10.1093/gerona/55.11.m691. doi: 10.1093/gerona/55.11.M691. [DOI] [PubMed] [Google Scholar]
- 10.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–22. doi: 10.1046/j.1532-5415.2003.51104.x. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 11.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Osir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A-Biol. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. doi: 10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- 12.Eng JJ, Chu KS, Dawson AS, Kim CM, Hepburn KE. Functional walk tests in individuals with stroke: relation to perceived exertion and myocardial exertion. Stroke. 2002;33:756–61. doi: 10.1161/hs0302.104195. doi: 10.1161/hs0302.104195. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 14.Lord SR, Menz HB. Physiologic, psychologic, and health predictors of 6-minute walk performance in older people. Arch Phys Med Rehabil. 2002;83:907–11. doi: 10.1053/apmr.2002.33227. doi: 10.1053/apmr.2002.33227. [DOI] [PubMed] [Google Scholar]
- 15.Chetta A, Zanini A, Pisi G, Aiello M, Tzani P, Neri M, et al. Reference values for the 6-min walk test in healthy subjects 20–50 years old. Respir Med. 2006;100:1573–8. doi: 10.1016/j.rmed.2006.01.001. doi: 10.1016/j.rmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Camarri B, Eastwood PR, Cecins NM, Thompson PJ, Jenkins S. Six-minute walk distance in healthy subjects aged 55–75. Respir Med. 2006;100:658–65. doi: 10.1016/j.rmed.2005.08.003. doi: 10.1016/j.rmed.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Hulens M, Vansant G, Claessens AL, Lysens R, Muls E. Predictors of 6-minute walk test results in lean, obese and morbidly obese women. Scand J Med Sci Sports. 2003;13:98–105. doi: 10.1034/j.1600-0838.2003.10273.x. doi: 10.1034/j.1600-0838.2003.10273.x. [DOI] [PubMed] [Google Scholar]
- 18.Esposito JG, Thomas SG, Kingdon L, Ezzat S. Anabolic growth hormone action improves submaximal measures of physical performance in patients with HIV-associated wasting. Am J Physiol-Endoc M. 2005;289:E494–503. doi: 10.1152/ajpendo.00013.2005. doi: 10.1152/ajpendo.00013.2005. [DOI] [PubMed] [Google Scholar]
- 19.Enright PL, Sherrill DL. Reference equations for six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–7. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 20.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6-minute walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–98. doi: 10.1378/chest.123.2.387. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 21.Troosters T, Gosselink R, Decramer M. Six-minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–4. doi: 10.1034/j.1399-3003.1999.14b06.x. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 22.Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001;21:87–93. doi: 10.1097/00008483-200103000-00005. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Mossberg KA. Reliability of a timed walk test in persons with acquired brain injury. Am J Phys Med Rehabil. 2003;82:385–90. doi: 10.1097/01.PHM.0000052589.96202.BE. doi: 10.1097/00002060-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Savci S, Inal-Ince D, Arikan H, Guclu-Gunduz A, Cetisli-Korkmaz N, Armutlu K, et al. Six-minute walk distance as a measure of functional exercise capacity in multiple sclerosis. Disabil Rehabil. 2005;27:1365–71. doi: 10.1080/09638280500164479. doi: 10.1080/09638280500164479. [DOI] [PubMed] [Google Scholar]
- 25.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. J Am Med Assoc. 1993;270:1702–7. doi: 10.1001/jama.270.14.1702. [PubMed] [Google Scholar]
- 26.Cahalin LP, Mathier MM, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in advanced heart failure. Chest. 1996;110:325–32. doi: 10.1378/chest.110.2.325. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM. Walking performance and cardiovascular response: associations with age and morbidity—The Health, Aging and Body Composition Study. J Gerontol A-Biol. 2003;58:715–20. doi: 10.1093/gerona/58.8.m715. doi: 10.1093/gerona/58.8.M715. [DOI] [PubMed] [Google Scholar]
- 28.Chetta A, Rampello A, Marangio E, Merlini S, Dazzi F, Aiello M, et al. Cardiorespiratory response to walk in multiple sclerosis patients. Respir Med. 2004;98:522–9. doi: 10.1016/j.rmed.2003.11.011. doi: 10.1016/j.rmed.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Paltamaa J, Sarasoja T, Leskinen E, Wikstrom J, Malkia E. Measuring deterioration in international classification of functioning domains of people with multiple sclerosis who are ambulatory. Phys Ther. 2008;88:176–90. doi: 10.2522/ptj.20070064. doi: 10.2522/ptj.20070064. [DOI] [PubMed] [Google Scholar]
- 30.Guralnik JM, LaCroix AZ, Abbott RD, Berkman LF, Satterfield S, Evans DA, et al. Maintaining mobility in late life, I: demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–57. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 31.Trojano M, Liguori M, Zimatore GB, Bugarini R, Avolio C, Paolicelli D, et al. Age-related disability in multiple sclerosis. Ann Neurol. 2002;51:475–80. doi: 10.1002/ana.10147. doi: 10.1002/ana.10147. [DOI] [PubMed] [Google Scholar]
- 32.Franklin BA, Whaley MH, editors. ACSM's guidelines for exercise testing and prescription. 6th ed. Philadelphia: American College of Sports Medicine; 2000. [Google Scholar]
- 33.Kurtzke JF. Rating neurological impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 34.Fry DK, Pfalzer LA. Reliability of four functional tests and rating of perceived exertion in persons with multiple sclerosis. Physiother Can. 2006;58:212–20. doi: 10.3138/ptc.58.3.212. [Google Scholar]
- 35.Yancik R, Wesley MN, Ries LA, Havlik RJ, Long S, Edwards BK, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82:2123–34. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2123::AID-CNCR6>3.3.CO;2-N. [PubMed] [Google Scholar]
- 36.Houterman S, Janssen-Heijnen MLG, Verheij CDGW, Louwman WJ, Vreugdenhil G, van der Sangen MJC, et al. Comorbidity has negligible impact on treatment and complications but influences survival in breast cancer patients. Brit J Cancer. 2004;90:2332–7. doi: 10.1038/sj.bjc.6601844. doi: 10.1038/sj.bjc.6601844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale. application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 38.Hatch J, Gill-Body KM, Portney LG. Determinants of balance confidence in community dwelling elderly people. Phys Ther. 2003;83:1072–9. [PubMed] [Google Scholar]
- 39.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) scale. J Gerontol A-Biol. 1995;50:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 40.Paltamaa J, West H, Sarasoja T, Wikstrom J, Malkia E. Reliability of physical functioning measures in ambulatory subjects with multiple sclerosis. Physiother Res Int. 2005;10:93–109. doi: 10.1002/pri.30. doi: 10.1002/pri.30. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez MD, Goldberg A, Alexander N. Decreased muscle strength relates to self-reported stooping, crouching, or kneeling difficulty in older adults. Phys Ther. 2010;90:67–74. doi: 10.2522/ptj.20090035. doi: 10.2522/ptj.20090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83:49–57. [PubMed] [Google Scholar]
- 43.Jones CJ, Rikkli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70:113–9. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 44.American Thoracic Society; European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 45.American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 46.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow volume curve normal standards variability and effects of age. Am Rev Respir Dis. 1976;113:587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 47.Cherniak RM, Rabner MD. Normal standards for ventilatory function using an automated wedge spirometer. Am Rev Respir Dis. 1972;106:38–46. doi: 10.1164/arrd.1972.106.1.38. [DOI] [PubMed] [Google Scholar]
- 48.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 49.Keppel G, Wickens T. Design and analysis: a researcher's handbook. Upper Saddle River (NJ): Pearson Prentice Hall; 2004. Correction for cumulative Type 1 error; pp. 176–81. [Google Scholar]
- 50.Perneger T. What's wrong with Bonferroni adjustments? Brit Med J. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 2nd ed. Upper Saddle River (NJ): Prentice Hall; 2000. [Google Scholar]
- 52.Lernier-Frankiel M, Vargas S, Brown M, Krusell L, Shoneberger W. Functional community ambulation: what are your criteria? Clin Manag Phys Ther. 1986;6:12–5. [Google Scholar]
- 53.Cohen JJ, Sveen JD, Walker JM, Brummel-Smith K. Establishing criteria for community ambulation. Top Ger Rehabil. 1983;1:71–7. [Google Scholar]
- 54.Robinett C, Vondran M. Functional ambulation velocity and distance requirements in rural and urban communities. Phys Ther. 1988;68:1371–3. doi: 10.1093/ptj/68.9.1371. [DOI] [PubMed] [Google Scholar]
- 55.Myers A, Powell L, Maki B, Holliday P, Brawley L, Sherk W. Psychological indicators of balance confidence: relationship to actual and perceived abilities. J Gerontol A-Biol. 1996;51:M37–43. doi: 10.1093/gerona/51a.1.m37. doi: 10.1093/gerona/51A.1.M37. [DOI] [PubMed] [Google Scholar]
- 56.Brouwer B, Musselman K, Culham E. Physical function and health status among seniors with and without a fear of falling. Gerontology. 2004;50:135–41. doi: 10.1159/000076771. doi: 10.1159/000076771. [DOI] [PubMed] [Google Scholar]
- 57.Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837–41. doi: 10.1016/s0003-9993(99)90236-8. doi: 10.1016/S0003-9993(99)90236-8. [DOI] [PubMed] [Google Scholar]
- 58.Thoumie P, Lamott D, Cantolloube S, Faucher M, Amarenco G. Motor determinants of gait in 100 ambulatory patients with multiple sclerosis. Mult Scler. 2005;11:485–91. doi: 10.1191/1352458505ms1176oa. doi: 10.1191/1352458505ms1176oa. [DOI] [PubMed] [Google Scholar]
- 59.Smeltzer S, Skurnick JH, Troiano R, Cook SD, Duran W, Lavietes MH. Respiratory function in multiple sclerosis: utility of clinical assessments of respiratory muscle function. Chest. 1992;101:479–84. doi: 10.1378/chest.101.2.479. doi: 10.1378/chest.101.2.479. [DOI] [PubMed] [Google Scholar]
- 60.Gosselink R, Kovacs L, Decramer M. Respiratory muscle involvement in multiple sclerosis. Eur Respir J. 1999;13:449–54. doi: 10.1183/09031936.99.13244999. doi: 10.1183/09031936.99.13244999. [DOI] [PubMed] [Google Scholar]
- 61.Buyse B, Demedts M, Meekers J, Vandegaer L, Rochette F, Kerkhofs L. Respiratory dysfunction in multiple sclerosis: a prospective analysis of 60 patients. Eur Respir J. 1997;10:139–45. doi: 10.1183/09031936.97.10010139. doi: 10.1183/09031936.97.10010139. [DOI] [PubMed] [Google Scholar]
- 62.Fry DK, Pfalzer LA, Chokski AR, Wagner MT, Jackson ES. Randomized control trial of effects of a 10-week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. J Neurol Phys Ther. 2007;31:162–72. doi: 10.1097/NPT.0b013e31815ce136. [DOI] [PubMed] [Google Scholar]
- 63.Chiara T, Martin D, Davenport PW, Bolser DC. Expiratory muscle strength training in persons with multiple sclerosis having mild to moderate disability: effect on maximal expiratory pressure, pulmonary function, and maximal voluntary cough. Arch Phys Med Rehabil. 2006;87:468–73. doi: 10.1016/j.apmr.2005.12.035. doi: 10.1016/j.apmr.2005.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smeltzer S, Lavietes MH, Cook SD. Expiratory training in multiple sclerosis. Arch Phys Med Rehabil. 1996;77:909–12. doi: 10.1016/s0003-9993(96)90281-6. doi: 10.1016/S0003-9993(96)90281-6. [DOI] [PubMed] [Google Scholar]
- 65.Kelfbeck B, Nedjad JH. Effect of inspiratory muscle training in patients with multiple sclerosis. Arch Phys Med Rehabil. 2003;84:994–9. doi: 10.1016/s0003-9993(03)00133-3. doi: 10.1016/S0003-9993(03)00133-3. [DOI] [PubMed] [Google Scholar]
- 66.Lord SE, Rochester L. Measurement of community ambulation after stroke. Stroke. 2005;36:1457–61. doi: 10.1161/01.STR.0000170698.20376.2e. doi: 10.1161/01.STR.0000170698.20376.2e. [DOI] [PubMed] [Google Scholar]
- 67.Shumway-Cook A, Patla AE, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental demands associated with community mobility in older adults with and without disability. Phys Ther. 2002;82:670–81. [PubMed] [Google Scholar]
- 68.Pang MYC, Eng JJ, Miller WC. Determinants of satisfaction with community reintegration in older adults with chronic stroke: role of balance self-efficacy. Phys Ther. 2007;87:1–10. doi: 10.2522/ptj.20060142. doi: 10.2522/ptj.20060142. [DOI] [PubMed] [Google Scholar]
- 69.Lie-Ambrose T, Khan KM, Eng JJ, Lord SR, McKay HA. Balance confidence improves with resistance or agility training: increase is not correlated with objective changes in fall risk and physical abilities. Gerontol. 2004;50:373–82. doi: 10.1159/000080175. doi: 10.1159/000080175. [DOI] [PMC free article] [PubMed] [Google Scholar]