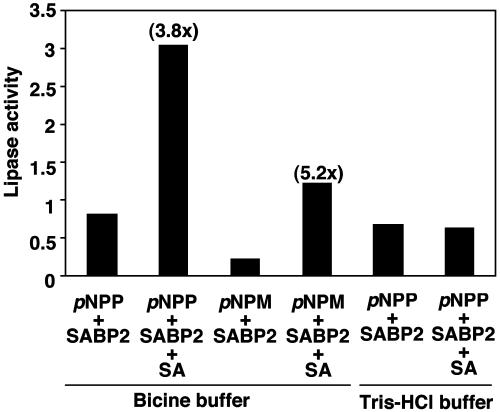
Fig. 3.
SA stimulates the lipase activity of recombinant SABP2. Results from lipase assays using pNP palmitate (para-nitrophenyl palmitate) as the substrate in the presence or absence of SA are presented as an average with three separate preparations of rSABP2, whereas those using pNP myristate (para-nitrophenyl myristate) as the substrate were done with one preparation of rSABP2. Lipase activity detected in each sample is presented in relative units, and fold stimulation by SA is shown in parentheses above the bars. One relative unit is the amount of enzyme that releases 0.017 μmol/min p-nitrophenol. Note that when 50 mM bicine is replaced with 50 mM Tris·HCl, pH 8.0, SABP2 does not bind SA.