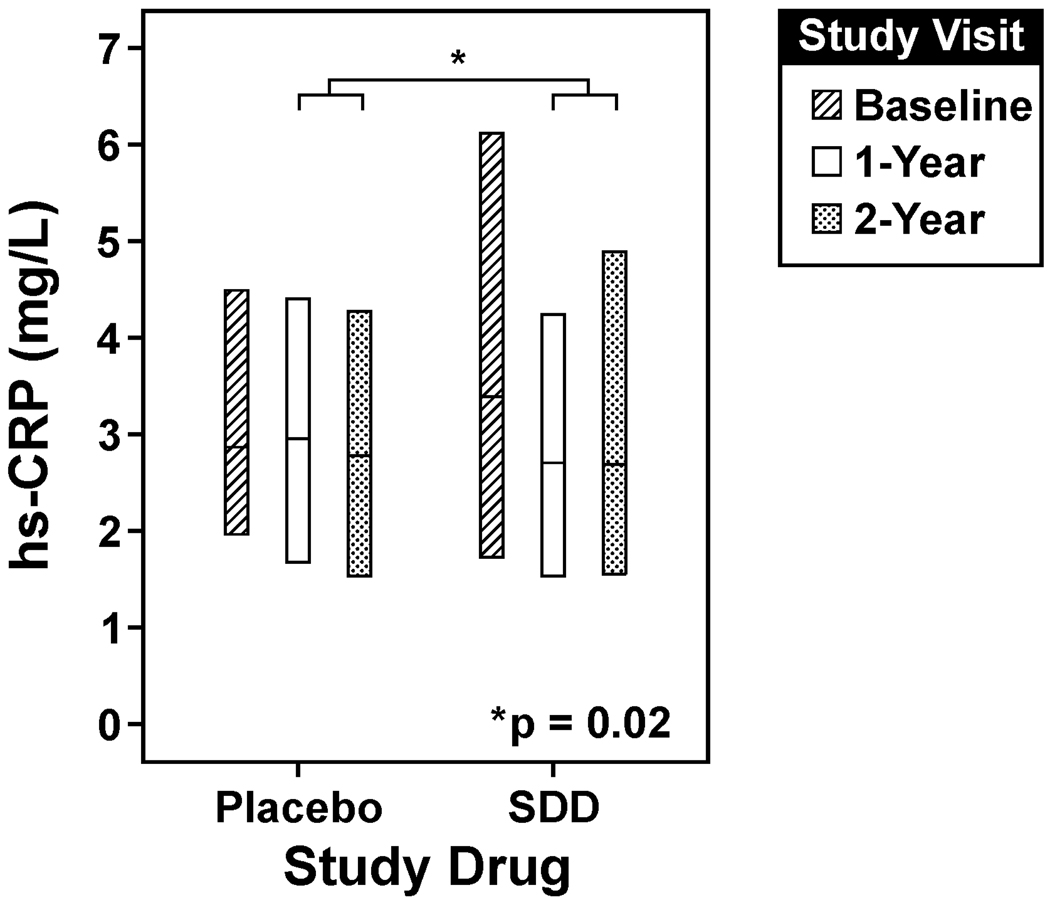
Figure 2. SDD effects on serum hs-CRP over the two-year clinical trial.
SDD reduced median hs-CRP levels by 18% compared to placebo over the two-year protocol after adjustment for baseline hs-CRP level, smoking status, study center, study visit and batch effects, which was statistically significant (p=0.02, intent-to-treat analysis). The lower edge of the box represents the 25th percentile of the observed distribution, while the center line and upper edge of the box represent the 50th and 75th percentiles of the observed distribution, respectively. The number of subjects analyzed at each time point in the placebo group is as follows: 62 at baseline, 61 at the 1-year visit (serum sample not available for one subject) and 62 at the two-year visit. 51 subjects were analyzed at each time point in the SDD group. hs-CRP = high-sensitivity C-reactive protein.