Abstract
Telomeres are noncoding DNA regions at the end of the chromosomes that are crucial for genome stability. Since telomere length decreases with cell division, they can be used as a signature of cell proliferation history. T-cell reconstitution in severe combined immunodeficiency (SCID) subjects, recipients of T-cell-depleted, allogeneic-related bone marrow cells, is due to the development and maturation of donor T-cell precursors in the infant’s vestigial thymus and to homeostatic proliferation of mature T cells in the peripheral organs. Since T-cell function, thymic output, and T-cell clonal diversity are maintained long term in these patients, we investigated whether donor T-cell engraftment resulted in increased telomere shortening. Our study of seven SCID patients, following successful bone marrow transplantation, demonstrates that the patients’ peripheral T cells did not exhibit greater than normal telomere shortening.
Keywords: SCID, Telomere, Transplantation, T cell, TREC
Telomeres consist of 5–15 kb of tandemly repeated (TTAGGG) n sequences and associated proteins present at the ends of all chromosomes, and they maintain the stability and integrity of the genome [1–3]. Due to incomplete terminal replication during DNA synthesis, telomeres shorten with each cell division. Loss of functional telomere length below a critical threshold can result in cell senescence or death [4]. Several studies in vitro reported that telomere length decreases by approximately 50–200 bp for each somatic cell division: this observation applies to lymphocytes and other somatic cells [3, 5]. Telomere length represents a balance between the loss of telomeric repeats, which occurs during cell division with incomplete DNA replication, and the addition of telomeric repeats by the RNA-dependent DNA polymerase, or telomerase [6, 7]. Telomerase is capable of synthesizing terminal telomeric sequences and compensating for the telomere shortening. Although activated lymphocytes express telomerase, the level of expression is not sufficient in vitro or in vivo to prevent telomere shortening during extensive cell division, ultimately triggering cell senescence [8].
Mature lymphocytes have a limited life span. The maintenance of sufficient number of lymphocytes depends on the production of new lymphocytes from stem cells and on their subsequent proliferation [9]. A number of studies have investigated the issue of telomere shortening in lymphocytes and other blood cells generated by stem cells in hematopoietic transplant recipients [10]. In adult leukemic patients, hematopoietic reconstitution after chemoablation and bone marrow transplantation (BMT) is associated with an increased stem cell proliferation and consequent telomere shortening in peripheral blood cells [11]. In addition, the number of transplanted cells appears to be inversely correlated with telomere shortening [10, 11]. While some of these studies report telomere shortening in transplant recipients, such shortening is often not significant enough to affect the result of hematopoietic transplantation.
Studies by our group demonstrated that T-cell reconstitution in severe combined immunodeficiency (SCID) subjects, recipients of T-cell-depleted allogeneic-related BM cells [12], is due to the development and maturation of donor T-cell precursors in the infant’s vestigial thymus [13]. Within the SCID thymus, donor T-cell precursors undergo T-cell receptor (TCR) gene rearrangements by the junction of V(D) J gene segments and by the addition of N nucleotides. The process of TCR rearrangement generates extra-chromosomal DNA episomes or TCR excision circles (TRECs), which can be detected in newly generated T cells. The presence of TRECs in circulating T cells is an indication that rearrangement of their TCR genes has recently occurred in the thymus. Because the frequency of TRECs is reduced by activation-induced proliferation of T cells, the maintenance of a high frequency of TREC is evidence of continued thymopoiesis. Continued thymopoiesis balanced with a normal proliferation of mature T cells maintains a diverse repertoire in the peripheral T-cell pool [14]. In a recently published 25-year follow-up study of 128 patients with 11 different molecular types of SCID after nonconditioned BMT, we provided evidence that T-cell function, thymic output, and T-cell clonal diversity are maintained long term in these patients [15]. However, we did not address the issue of whether donor T-cell engraftment in these patients caused an increased proliferation of donor T cells ultimately resulting in a greater-than-normal telomere shortening.
Here, we analyzed seven SCID subjects (four X-linked, two Jak3, and one IL-7R) from our cohort, all of whom had normal TREC values over time (Fig. 1) [15], and we examined the telomere length of T-cell subpopulations for each subject. Telomeres were measured using a modification of the flow-FISH technique [16], which allows simultaneous fluorescent immunophenotyping of cells by quantum dot-labeled antibodies and telomere hybridization using a fluorescent peptide nucleic acid (PNA) probe complementary to the telomere terminal repeat sequence, as described by Kapoor et al. [17]. This method allows for the analysis of small numbers of specific cell subset without the need for cell sorting. As normal control, we used T cells from cord blood and an adult, 40-year-old subject samples. Each experimental sample was analyzed in the presence of specific internal controls, such as calf thymocytes (long telomere length = 23 Kb) and Jurkat cells (short telomere length = 6 Kb).
Fig. 1.
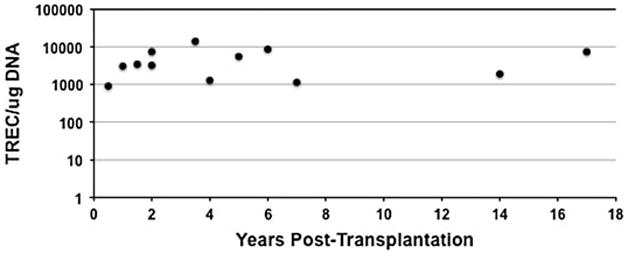
Analysis of thymic function over time after BMT in all SCIDs. TREC values were observed over time in seven subjects from whom samples were available for analysis, each subject associated with one of three molecular types of SCID. The points on the figure represent the TREC values for each of four X-linked SCID subjects tested between 0.5 and 7 years post-BMT, two Jak3 SCID subjects tested between 3.5 and 17 years post-BMT, and one IL-7R SCID subject tested between 1.5 and 5 years post-BMT
We analyzed CD3+, CD3+CD4+, CD3+CD8+, CD3+CD4+CD45RA+ or CD45RA– and CD3+CD8+CD45RA+or CD45RA– T-cell subsets from all seven SCID subjects and control peripheral blood mononuclear cell (PBMC) samples. As shown in Fig. 2, T-cell subsets from SCID subjects’ samples, at early (0.5 years) and later (17 years) time following BMT, maintained telomere length within the normal ranges of control samples. In fact, X-linked 3 (7), Jak3 1 (3.5), Jak3 2 (17), and IL-7R 1 (1.5) subjects had the lowest telomere length, but their samples were collected at early (1.5, 3.5), medium (7), and late (17) years post-transplantation, suggesting that time following BMT did not directly correlate with telomere shortening in this cohort of subjects. As previously reported by others, both CD4+-naïve and CD8+-naïve CD45RA+ had longer telomeres than effector/memory CD45RA− T cells in all subjects and controls [18, 19]. Subject Jak3 2 (17) had the lowest telomere length of all subjects in both CD3+CD4+CD45RA-(8.43 Kd) and CD3+CD4+CD45RA- (9.39 Kd), followed by Jak3 2 (14) with CD3+CD4+CD45RA- (9.92 Kb) and IL-7R 1 (1.5) with CD3+CD8+CD45RA-(9.52 Kb), again suggesting that time post-BMT did not correlate with telomere shortening. We did not detect consistent differences in the telomere length of CD4+ versus CD8+ T-cell subsets reported by others [18, 20]. Differences between molecular types of SCIDs did not appear greater than the subject-to-subject variability. Further studies will need to be performed to analyze the telomere length in larger numbers of subjects and in those SCIDs, like the RAG-1- and -2-deficient subjects, whose T-cell reconstitution was less successful than other molecular types of SCIDs, following BMT [15, 21].
Fig. 2.
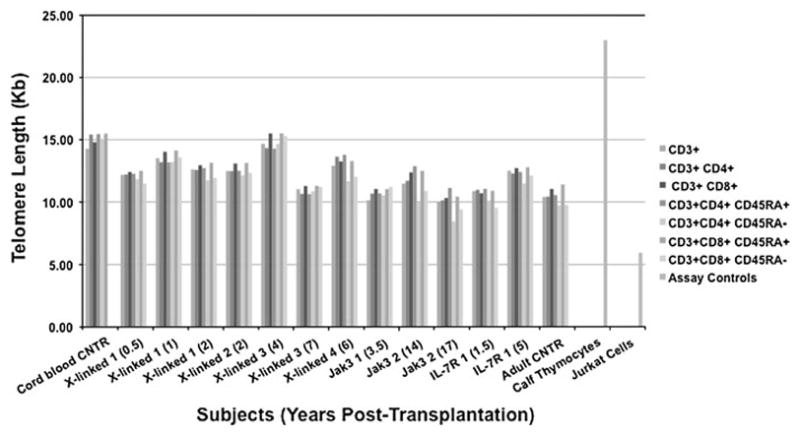
Telomere length in T-cell subsets of SCID subjects after BMT. Telomere length, measured by flow-FISH in cells simultaneously stained by quantum dot-labeled antibodies, was determined by flow cytometry on PBMC collected from seven SCID subjects at the indicated times post-BMT (in parentheses)
In conclusion, although the number of subjects studied was small, these results indicate that SCID patients, following bone marrow transplantation, not only maintained normal thymic output over time, as shown in our earlier studies, but also did not have increased telomere shortening over controls in the T-cell subsets analyzed. This suggests that donor T cells in SCID recipients with normal TREC function are not short-lived.
Acknowledgments
We thank the patients and their families, V. Kapoor and W. G. Telford for the training on the telomere assay, P. M. Lansdorp for helpful advice on the Flow-FISH assay, R. Parrot and Z. Marshall for preparing and characterizing BM and PBMC, the Veterinary Staff at Martin S. Abattoir (Godwin, NC) for providing us with the calf thymus, and the Duke Human Vaccine Institute Flow Cytometry Facility. This study was supported by National Institutes of Health grant AI47605.
Contributor Information
Marcella Sarzotti-Kelsoe, Email: msarzott@duke.edu, Department of Immunology, Duke University Medical Center, Durham, NC 27710, USA, Department of Surgery, Duke University Medical Center, Durham, NC 27710, USA.
Xiaoju G. Daniell, Department of Surgery, Duke University Medical Center, Durham, NC 27710, USA
John F. Whitesides, Department of Medicine, Duke University Medical Center, Durham, NC 27710, USA
Rebecca H. Buckley, Department of Immunology, Duke University Medical Center, Durham, NC 27710, USA, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA
References
- 1.Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;1:245–55. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–13. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 3.Lansdorp PM. Major cutbacks at chromosome ends. Trends Biochem. 2005;30:388–95. doi: 10.1016/j.tibs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Harley CB, Futcher AB, Greiner CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 5.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–56. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 6.Hathcock KS, Chiang YJ, Hodes RJ. In vivo regulation of telomerase activity and telomere length. Immunol Rev. 2005;205:104–13. doi: 10.1111/j.0105-2896.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Zhi W, Wareski P, Weng N. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8 + T cells in vitro. J Immunol. 2005;174:4019–24. doi: 10.4049/jimmunol.174.7.4019. [DOI] [PubMed] [Google Scholar]
- 8.Lansdorp PM. Telomeres, stem cells, and hematology. Blood. 2008;111:1759–66. doi: 10.1182/blood-2007-09-084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marleau AM, Sarvetnick N. T cell homeostasis in tolerance and immunity. J Leukoc Biol. 2005;78:575–84. doi: 10.1189/jlb.0105050. [DOI] [PubMed] [Google Scholar]
- 10.Awaya N, Baerlocher GM, Manley TJ, et al. Telomere shortening in hematopoietic stem cell transplantation: a potential mechanism for late graft failure? Biol Blood Marrow Transplant. 2002;8:597–600. doi: 10.1053/bbmt.2002.v8.abbmt080597. [DOI] [PubMed] [Google Scholar]
- 11.Notaro R, Cimmino A, Tabarini D, et al. In vivo telomere dynamics of human hematopoietic stem cells. PNAS. 1997;94:13782–5. doi: 10.1073/pnas.94.25.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley RH, Schiff SE, Schiff RI, et al. Hematopoietic stem cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–16. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 13.Patel DD, Gooding ME, Parrott RE, et al. Thymic function after hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 2000;342:1325–32. doi: 10.1056/NEJM200005043421804. [DOI] [PubMed] [Google Scholar]
- 14.Sarzotti M, Patel DD, Li X, et al. T cell repertoire development in humans with SCID after nonablative allogeneic marrow transplantation. J Immunol. 2003;170:2711–8. doi: 10.4049/jimmunol.170.5.2711. [DOI] [PubMed] [Google Scholar]
- 15.Sarzotti-Kelsoe M, Win CM, Parrott RE, et al. Thymic output, T cell diversity and T cell function in long-term human SCID Chimeras. Blood. 2009;114:1445–53. doi: 10.1182/blood-2009-01-199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baerlocher GM, Vulto I, de Jong G, Lansdorp Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1:2365–76. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor V, Hakim FT, Rehman N, et al. Quantum dots thermal stability improves simultaneous phenotype-specific telomere length measurement by FISH-flow cytometry. J Immunol Meth. 2009;344:6–14. doi: 10.1016/j.jim.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rufer N, Brummendorf TH, Kolvraa S, et al. Telomere fluorescence Measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–67. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghans JA, Bredius RG, Hazenberg MD, et al. Early determinants of long-term T-cell reconstitution after hematopoietic stem cell transplantation for severe combined immunodeficiency. Blood. 2006;108:763–9. doi: 10.1182/blood-2006-01-009241. [DOI] [PubMed] [Google Scholar]
- 20.Palmer LD, Weng N, Levine B, et al. Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4 + and CD8 + T cells from HIV-discordant monozygotic twins. J Exp Med. 1997;185:1381–6. doi: 10.1084/jem.185.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz K, Gauss GH, Ludwig L, et al. RAG mutations in human B cell-negative SCID. Science. 1996;274:97–9. doi: 10.1126/science.274.5284.97. [DOI] [PubMed] [Google Scholar]


