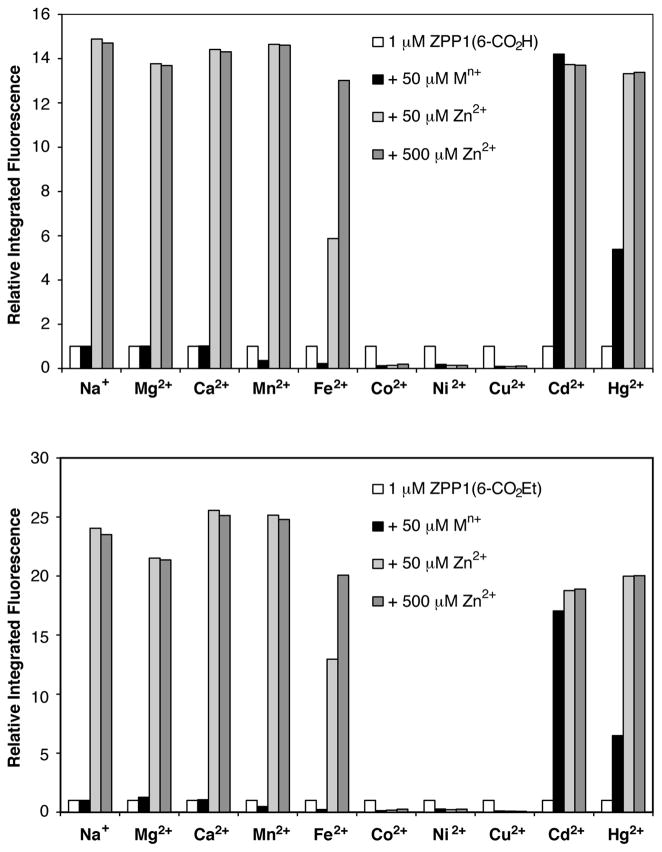
Figure 5.
Metal selectivity of ZPP1(6-CO2H) and ZPP1(6-CO2Et) in aqueous buffer at pH 7.0. For each sample, a 1 μM solution of the sensor (white bar) was mixed with 50 μM of the cation of interest (black bar), and then subsequently treated with 50 μM (light gray) or 500 μM (dark gray) ZnCl2. Integrated fluorescence after each addition is normalized with respect to the fluorescence of the metal-free sensor. λex = 505 nm for ZPP1(6-CO2H), λex = 508 nm for ZPP1(6-CO2Et)