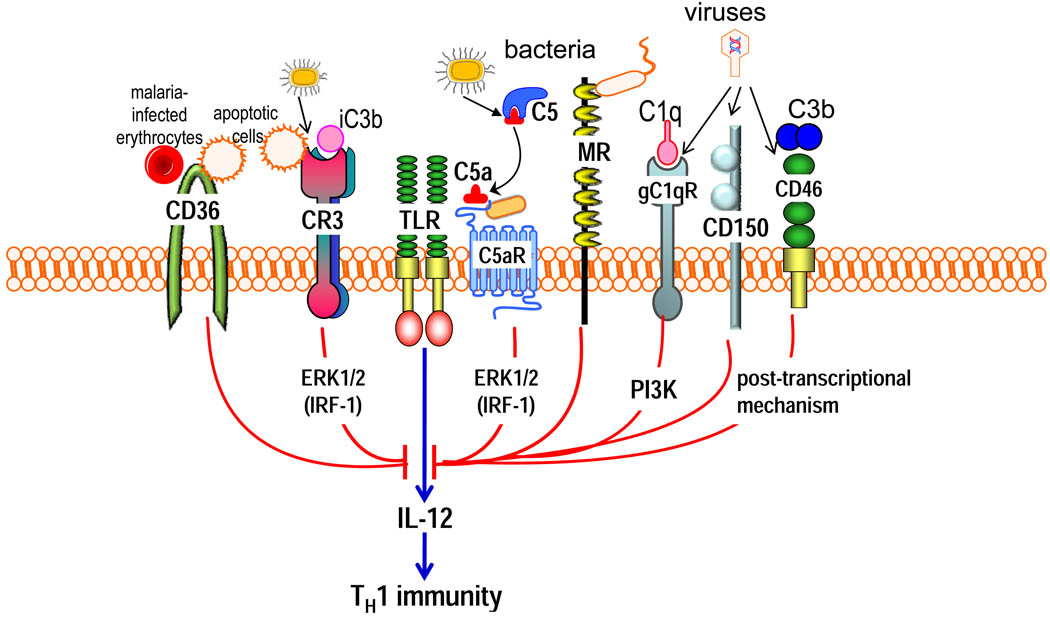
Figure 5. Selective inhibition of TLR-induced IL-12 production by pathogen-instigated PRR crosstalk.
The crosstalk between anaphylatoxin receptors (particularly C5a receptor [C5aR]) or other complement receptors (such as complement receptor-3 [CR3], gC1q receptor [gC1qR] and CD46) and Toll-like receptors (TLRs) selectively inhibits the induction of IL-12. Relatively little is known regarding the pathways mediating this selective inhibition; signalling molecules that have been implicated, such as extracellular signal-regulated kinase-1 (ERK1) and ERK2 and phosphatidylinositol-3 kinase (PI3K), are shown downstream of the corresponding receptors. At least for ERK1 and ERK2, the selectivity of IL-12 inhibition is attributed to the suppression of a crucial transcription factor, the interferon regulatory factor 1 (IRF1). Posttranscriptional mechanisms might also contribute to IL-12 inhibition. Activation of these complement receptors, or other innate immune receptors (such as CD36, mannose receptor and CD150) that share the ability to downregulate IL-12, by their natural ligands might have a homeostatic function. However, these same receptors can be activated by bacterial, viral or parasitic pathogens, which can thereby downregulate TLR-induced IL-12 production to interfere with host defences (such as the inhibition of TH1 cell-mediated immunity). Although microbial molecules that act as ligands for C5aR have been described, this receptor can come under pathogen control also through the enzymatic generation of high levels of C5a by microbial C5 convertase-like enzymes.