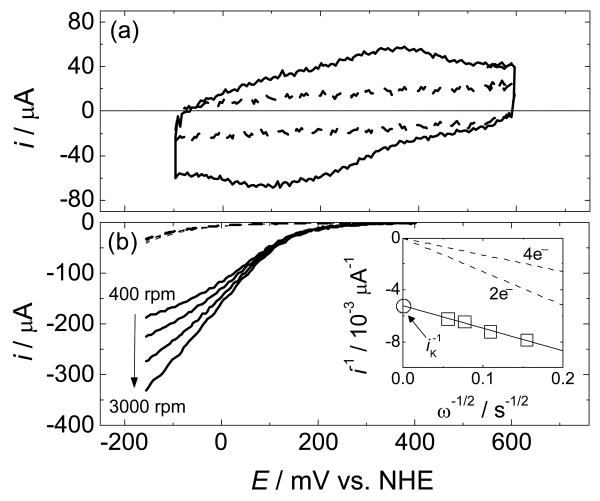
Figure 1.
(a) Cyclic voltammogram (CV) of Cu(phenC) on a static glassy carbon electrode in an Ar-purged aqueous solution (scan rate = 1000 mV/s). The dashed line is the same CV repeated after Cu was removed by exposing the surface to a Cu chelating agent for 20 min while rotating the electrode at 3000 rpm. (b) Rotating-disk voltammograms for the reduction of O2 in an O2-saturated aqueous solution by Cu(phenC) (scan rate = 25 mV/s). The dashed lines are the same set of rotating-disk voltammograms repeated after Cu was removed by exposing the surface to a copper-chelating agent for 20 min while rotating the electrode at 3000 rpm. The inset is a Koutecky-Levich plot of the inverse of the disk current measured at 0 mV vs. NHE as a function of square root of the inverse of the rotation rate. The dashed lines are calculated diffusion-limited currents for the reduction of O2 by 2 and 4 electrons. The fitted line yields n = 3.6 electrons. The intercept is the inverse of the kinetically limited current, (iK)-1. All solutions contained 0.05M sodium acetate, 0.05M acetic acid, 1 M sodium perchlorate with measured pH = 4.8.