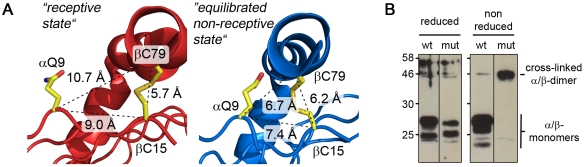
Figure 4. Validation of the helical mobility by experimental introduction of an intermolecular disulfide bond.
A, Postulated positions of residues αQ9 and βC79 in the receptive and the non-receptive state. The backbone of the α- and the β-chain is shown in red (receptive) and blue (non-receptive), respectively. The perspective is chosen to point into the binding cleft near the P1 pocket, with the β1 α-helix forming the right boundary. Side chains are shown for residues αQ9 and βC79 as well as for βC15, naturally forming an intrachain disulfide bridge with βC79. The Cα-distances are indicated. The receptive state is shown on the left side and the non-receptive state, characterized by a shift of the α-helix towards the center of the cleft, on the right side. B, SDS-PAGE analysis of the αQ9C/βC15S mutant. Lysates of cells expressing either HLA-DR1 (wt) or the double mutant αQ9C/βC15S (mut) were separated by SDS-PAGE under reducing (left panel) and non-reducing conditions (right panel). The detection of bands representing either the single or the cross-linked α- and β-chains of HLA-DR1 was done by Western blot using polyclonal HLA-DR-specific serum. Apparent molecular weight and positions of molecular weight markers (in kDa) are indicated.