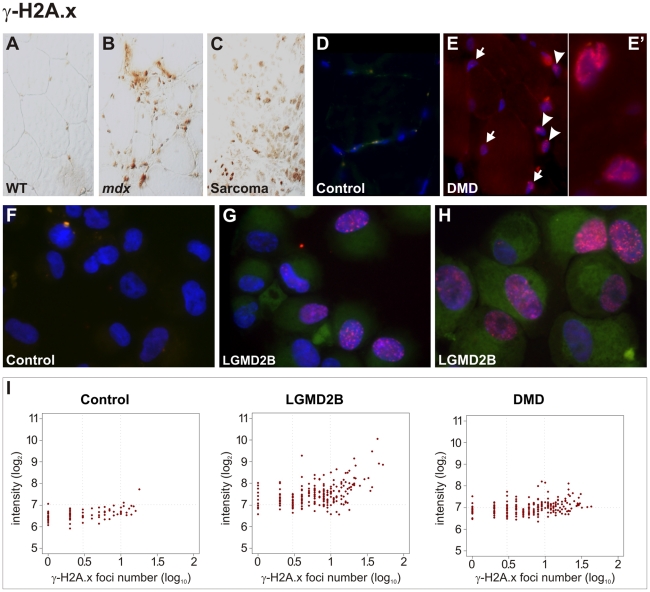
Figure 5. Pronounced DNA damage response in murine and human dystrophic muscle.
(A–C) Intense immunoreactivity with an antibody detecting Ser139-phosphorlated histone H2A.x (γ-H2A.x) in skeletal muscle from a dystrophin-deficient (mdx) mouse (B) and in a sarcoma from a mdx mouse as well (C), in contrast to wild-type (WT) muscle (A). (D–E) In contrast to a healthy control (D), γ-H2A.x immunostainings revealed high levels of DSBs in muscle biopsies from DMD patients (E), with multiple nucleoplasmic γ-H2A.x foci formation in nuclei belonging both to muscle fibres (arrows) and non-muscle cells (arrow-heads). Close-up panel (E') shows two nuclei with intense foci formation. (F–H) Pronounced accumulation of γ-H2A.x foci in cultured myoblasts from LGMD2B patients (G, “362/03” and H, “90/01”), in contrast to myoblasts from a healthy donor (F, “363/07”). (I) Quantification of γ-H2A.x immunoreactivity revealed increased total reactivity and a prominent increase of cells with multiple (≥3) foci (log10 = 0.5) in myoblasts from LGMD2B (“362/03”) and DMD (“88/07”) patients compared to a control myoblast line (“363/07”). Fluorescence intensity is shown in a log2 scale, foci number is log10.