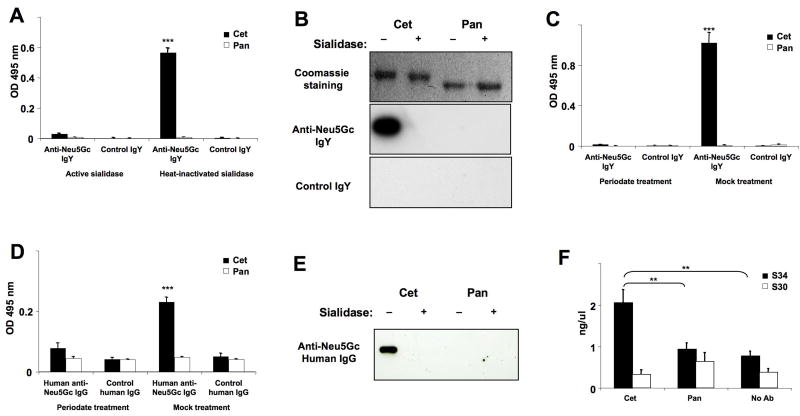
Figure 1. ELISA and Western-Blot Detection of Neu5Gc on Biotherapeutic Antibodies by Anti-Neu5Gc IgY Antibodies from Chickens or IgG Antibodies from Normal Human Serum.
Cetuximab (Cet) and Panitumumab (Pan) were treated with active sialidase to eliminate Sia epitopes or with heat-inactivated sialidase as control. Samples were used for ELISA (A) or Western Blot (B), in which Neu5Gc was detected using an affinity-purified chicken anti-Neu5Gc IgY or control IgY. In an additional ELISA (C), Cet and Pan were used for coating, then blocked, and sialic acid epitopes eliminated chemically using sodium metaperiodate. The reaction was stopped with sodium borohydride. As a control, periodate and borohydride were pre-mixed and then added to the wells (the borohydride inactivates the periodate). ELISA samples were studied at least in triplicate and data shown are Mean +/- SD. ***p <0.001, Paired Two-tailed t-test. (D) Cet and Pan were treated with sialidase or heat-inactivated sialidase as in Figure 1A and used for coating ELISA wells, then blocked and incubated with human anti-Neu5Gc IgG that had been purified from the serum of healthy humans and biotinylated. Samples were studied in triplicate and data shown as Mean +/- SD. ***p <0.001 (E) Cet and Pan (1 μg each) were separated by SDS-PAGE and Coomassie stained or blotted (see Figure 1B). Neu5Gc content was detected by using biotinylated human anti-Neu5Gc IgG. (F) Immune complex formation with Cet or Pan in whole human serum was detected using the CIC (C1Q) ELISA Kit (Buehlmann) as described in the manufacturer's guidelines. The absorbance was measured at 405 nm. Samples were studied in triplicate and data are shown as Mean +/- SD. **p <0.01, Paired Two-tailed t-test. Gels in Panels B and E were cropped for clarity of presentation. Full-length blots/gels are presented in Supplementary Figures 1-4.