Figure 2. Coupling of the folding energy landscape with the PN.
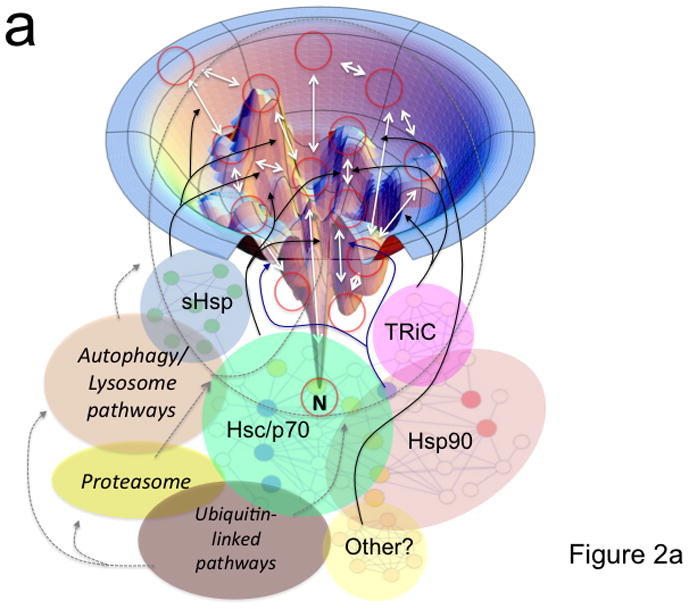
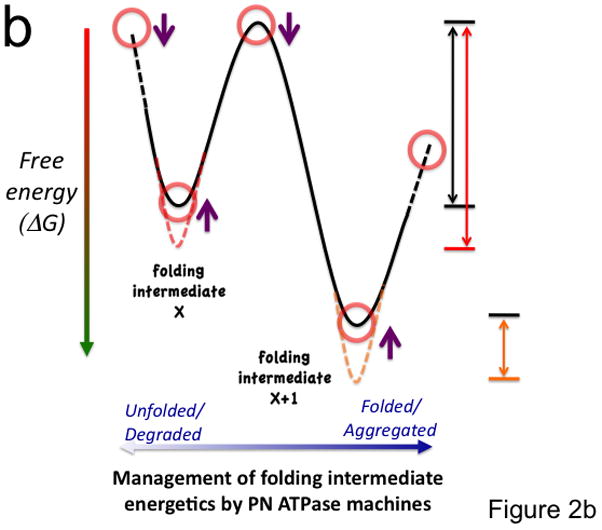
(a) Illustrated is a bumpy energy landscape funnel (http://www.dillgroup.ucsf.edu/) in which an unfolded protein proceeds along various intermediate steps (red circles) that can pose energetic barriers to achieve the native state (N) at the base of the funnel. The white arrows indicate potential pathways through various folding intermediates that the nascent protein may take to reach the native state. The solid black and blue (synthesis and folding modules) and the dashed gray (degradative module) arrows illustrate how different components of the PN may influence pathway choice. The dashed oval indicates that all intermediates are potential steps in which a protein can be targeted for degradation. Hsp90 is thought to principally facilitate late folding events (solid blue lines). (b) The energy landscape (a slice through the funnel illustrated in panel a) illustrates the central role of the ATPase cycle in synthesis, folding and degradative modules in managing the biology of the protein structure in the cell to achieve function. By coupling the energy of ATP hydrolysis (X-axis) by PN ATPase machines with the energetics imparted in the chemistry of the amino acid sequence of the polypeptide chain (Y-axis), PN ATPases maintain the protein fold in a dynamic state- essential for biology. Right side of panel illustrates energy barriers necessary to achieve a functional fold (black arrow) relative to the energetics associated with misfolding (red and orange arrows). The additional energetic demands challenging GPC through misfolding (red line) or aggregation (short orange line) are illustrated. Purple arrows indicate potential steps for GPC to alter folding kinetics and energetics to promote proteome balance and cell health. Abbreviations: sHsp (small heat shock proteins); TRiC (TCP1-ring complex).
