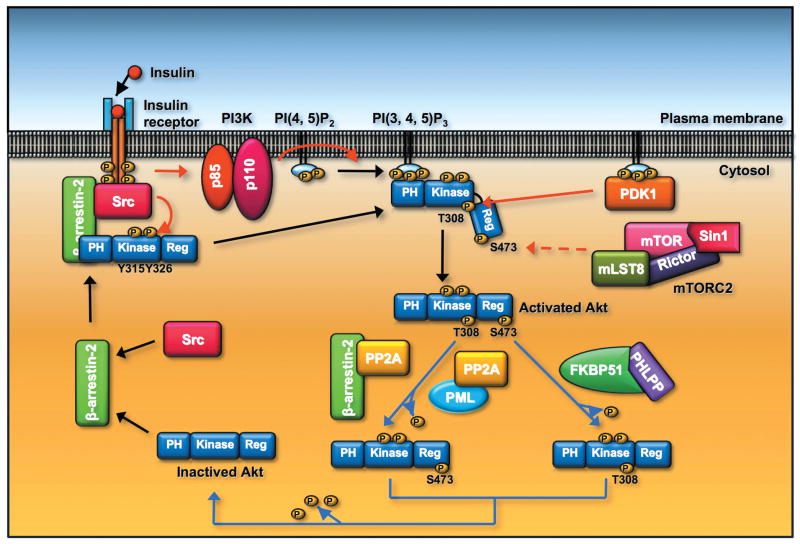
Figure 3.
The activity of Akt is regulated by phosphorylation and dephosphorylation of Akt. β-arrestin-2 recruits Src and Akt to the activated insulin receptor, and phosphoryaltion of Akt at Y315 and Y326 by Src is a prerequisite for Akt T308 and S473 phosphorylaiton induced by PDK1 and mTOC2, respectively, leading to the full activation of Akt. Akt T308 dephosphorylation is triggered by PP2A phosphatase. PML or β-arrestin-2 recruits PP2A and facilitates Akt T308 dephosphorylation. Dephosphorylation of Akt at S473 is induced by PHLPP phosphates. The adaptor protein FKBP51 recruits PHLPP to elicit Akt dephosphorylation at S473.