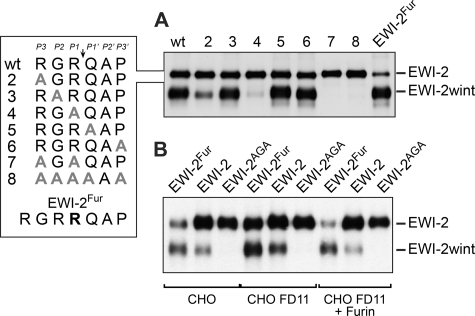
FIGURE 1.
Cleavage of EWI-2 leading to EWI-2wint production. A, wild type FLAG-tagged EWI-2 protein (wt) and proteins with mutations in the putative cleavage site (see box) were expressed in CHO cells. Cells were surface biotinylated and the proteins were precipitated with the M2 anti-FLAG antibody. Proteins were separated by SDS-PAGE under nonreducing conditions and detected using HRP-streptavidin. Expression of EWI-2 (wt) in CHO cells leads to the production of EWI-2 and EWI-2wint. B, wild type FLAG-tagged EWI-2 protein (EWI-2) or EWI-2 containing a furin cleavage consensus site (EWI-2Fur) or EWI-2 in which the RXR consensus site was mutated (EWI-2AGA, mutant 7 in A) were expressed either in regular CHO cells or CHO cells that were deficient for furin expression (CHO FD11), or CHO FD11 cells that overexpressed furin (CHO FD11 + Furin). Proteins were surface biotinylated, immunoprecipitated with M2 anti-FLAG antibody, separated by SDS-PAGE under nonreducing conditions, transferred to a nitrocellulose membrane, and detected using HRP-streptavidin.