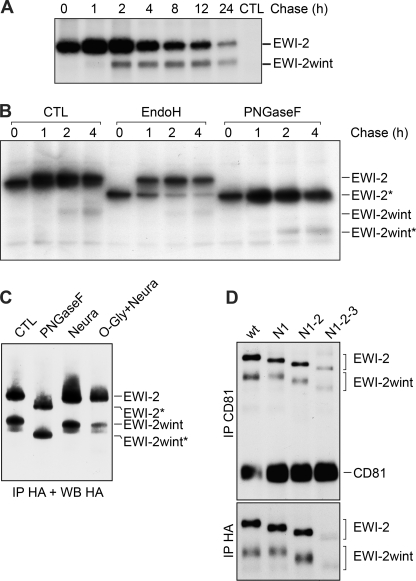
FIGURE 2.
Kinetics of production and glycosylation of EWI-2/EWI-2wint. A, CHO cells expressing C-terminal FLAG-tagged EWI-2 were pulse-labeled for 10 min with 35S-Protein Labeling Mix. After the indicated time of chase, cells were lysed in PBS, 1% Triton X-100 and proteins were precipitated with M2 antibody, separated by SDS-PAGE under nonreducing conditions, and detected by autoradiography. B, cells were labeled and lysed as described in A, after immunoprecipitation (IP) the proteins were either left untreated (CTL), treated with Endo H to remove high-mannose type glycans or treated with PNGase F to remove all N-linked glycosylations. Deglycosylated proteins are indicated by an asterisk. C, C-terminal HA-tagged EWI-2 proteins were expressed in CHO cells and precipitated using HA11 antibody. After precipitation, the samples were either left untreated (CTL), treated with PNGase F, or treated with neuraminidase (Neura) or O-glycanase and neuraminidase (O-Gly + Neura). Digested samples were resolved by SDS-PAGE and immunoblotted with the 3F10 anti-HA antibody. Deglycosylated proteins are indicated by an asterisk. D, expression of the glycosylation mutants of EWI-2 in which the potentially glycosylated Asn in Ig-domains one, three, or four were replaced by Gln, either only in Ig1 (N1), in Ig1 and Ig3 (N1–2), or all (N1–2-3). HA-tagged EWI-2 and glycosylation mutants were co-expressed with CD81 in CHO cells. Surface proteins were biotinylated and precipitated using HA11 anti-HA or 5A6 anti-CD81 antibodies. Proteins were detected using HRP-streptavidin.