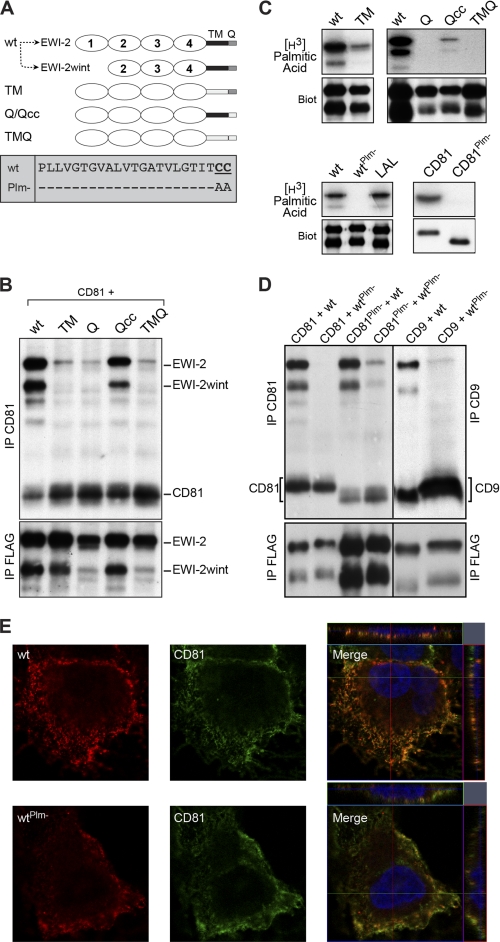
FIGURE 4.
Transmembrane domain and palmitoylation of EWI-2/EWI-2wint are important for the interaction with CD81. A, schematic representation of the different EWI-2 chimeric proteins and palmitoylation mutant. Expression of EWI-2 (wt) in CHO cells leads to the production of EWI-2 and EWI-2wint. B, FLAG-tagged chimeras of EWI-2 containing the transmembrane domain (TM) and/or the cytosolic tail (Q) of MHC II were co-expressed with CD81 in CHO cells. Surface-biotinylated proteins were lysed in PBS/Brij97/EDTA, immunoprecipitated, separated by SDS-PAGE under nonreducing conditions, and detected using HRP-streptavidin. C, metabolic labeling of EWI-2/EWI-2wint and CD81 mutants using [3H]palmitic acid, followed by IP. Expression of each protein was controlled by cell surface biotinylation (Biot). D, wild type proteins and palmitoylation-deficient mutants were co-expressed in CHO cells, biotinylated, lysed in PBS/Brij97/EDTA, and immunoprecipitated. Proteins were resolved by SDS-PAGE under nonreducing conditions and detected using HRP-streptavidin. In all experiments, anti-CD81, anti-CD9, and anti-FLAG IPs were performed with 5A6, SYB-I, and M2 antibodies, respectively. E, Huh-7 cells stably expressing either wild type EWI-2/EWI-2wint (wt) or palmitoylation mutant (wtPlm-) were labeled with antibodies to CD81 or HA epitope present at the N terminus of the EWI-2/EWI-2wint construct. Cells were imaged with an LSM710 confocal microscope using a ×63 oil immersion objective with a 1.4 numerical aperture. Representative confocal images are shown. Confocal sections of the Z-stack along the indicated lines are shown on the top and the right of merged images.