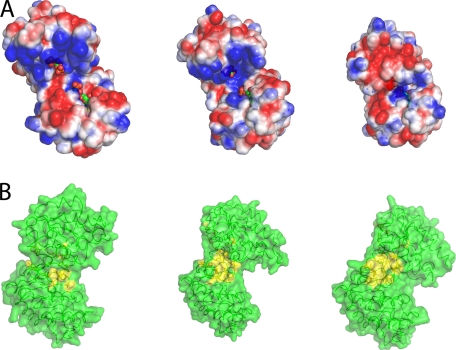
FIGURE 4.
Changes in the solvent exposed surface upon domain closure. A, electrostatic potential representation (blue, positive; red, negative; white, neutral; values from +2 kcal/mol to −2 kcal/mol) of the solvent accessible surface (probe radius, 1.4 Å) of the fully open apoenzyme conformation, the half-open binary complex, and the fully closed conformation. In the fully open conformation, the binding sites for ligands are well separated and exposed to the bulk solvent. The complementary charged areas that stabilize the closed conformation can be seen easily. Ligands are shown as spheres (green carbon atoms). B, surface representation of the transition from fully open to fully closed state. Residues involved in hydrophobic interactions are colored yellow. As the enzyme moves to the fully closed state, a hydrophobic patch is exposed.