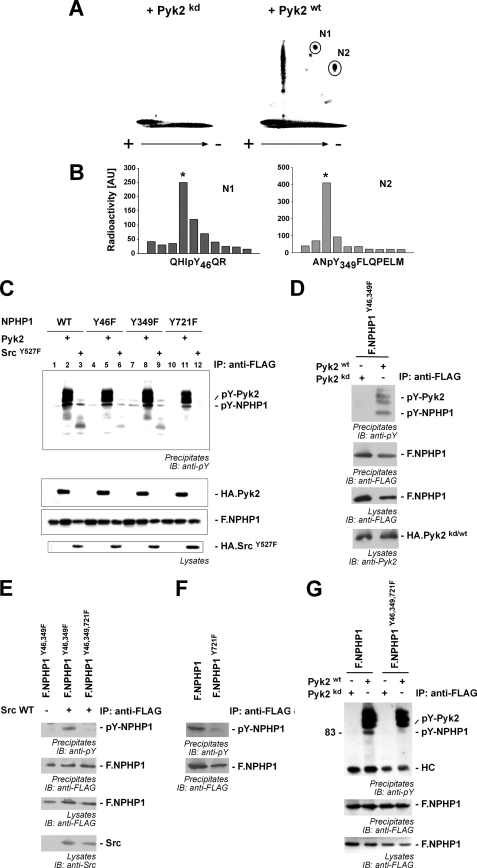
FIGURE 3.
Pyk2 induces NPHP1 phosphorylation of tyrosines 46, 349, and 721. A, transiently transfected HEK293T cells were labeled with 32P and lysed, and F.NPHP1 was immunoprecipitated. Precipitated NPHP1 was separated by SDS-PAGE, transferred to nitrocellulose membranes, and digested in situ with trypsin, and the peptides were separated on TLC plates by high voltage electrophoresis and ascending chromatography. + and − indicate voltage during electrophoresis. Separated phosphopeptides were visualized by autoradiography. Two Pyk2-dependent phosphopeptide fragments could be identified as phosphotyrosine-containing peptides (N1 and N2, marked with circles). B, phosphopeptides N1 and N2 were analyzed by Edman degradation which identified the position of the phosphorylated tyrosine residue in the peptide. C, tyrosine residues 46, 349, and 721 in NPHP1 were mutated to phenylalanine residues, and FLAG-tagged versions of these single mutants were co-expressed with empty vector (lanes 1, 4, 7, and 10), HA-tagged Pyk2 (lanes 2, 5, 8, and 11) or SrcY527F, a constitutively active mutant of c-Src (lanes 3, 6, 9, and 12). NPHP1 was precipitated and analyzed for tyrosine phosphorylation (upper panel). D, combined mutation of tyrosine residues 46 and 349 does not abrogate Pyk2-induced phosphorylation. Double mutant NPHP1 Y46F/Y349F was precipitated (IP), and tyrosine phosphorylation was monitored in presence of wild-type Pyk2 or a kinase-dead mutant. IB, immunoblotting. E, wild-type (WT) c-Src induces phosphorylation of the NPHP1 Y46F/Y349F double mutant, but not of the NPHP1 Y46F/Y349F/Y721F triple mutant. NPHP1 proteins were precipitated, and tyrosine phosphorylation was monitored. F, tyrosine residue 721 is endogenously phosphorylated. HEK293T cells were transfected and incubated for 15 min with peroxovanadate to inhibit tyrosine dephosphorylation. After cell lysis, NPHP1 proteins were precipitated and stained for phosphotyrosine. Mutation of Tyr-721 attenuated tyrosine phosphorylation of NPHP1. G, combined mutation of the mapped tyrosine residues abrogates tyrosine phosphorylation of NPHP1. NPHP1 proteins were precipitated, and tyrosine phosphorylation was monitored, revealing tyrosine phosphorylation of WT NPHP1 but not of the triple mutant. All immunoprecipitation experiments were performed at least three times.