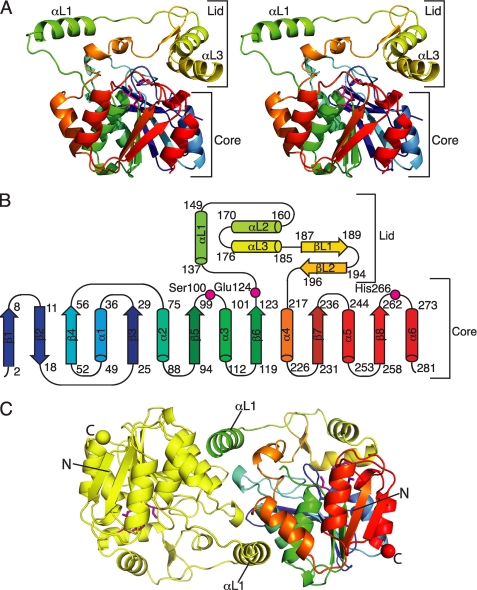
FIGURE 2.
Structure of curacin A thioesterase. A, CurM TE polypeptide. The stereo ribbon diagram is colored as a rainbow from blue at the N terminus to red at the C terminus with the catalytic triad residues in stick form with a magenta C. B, topology diagram. CurM TE has an α/β-hydrolase fold in the core domain and a novel lid topology. Residues of the catalytic triad (Ser100, Glu124, and His266) are labeled. C, backbone trace of the CurM TE dimer viewed along the molecular dyad. Monomers are colored as a rainbow (right) and in yellow (left), with the catalytic triad as in A, and N and C termini shown as spheres of the same color as the terminal residue.