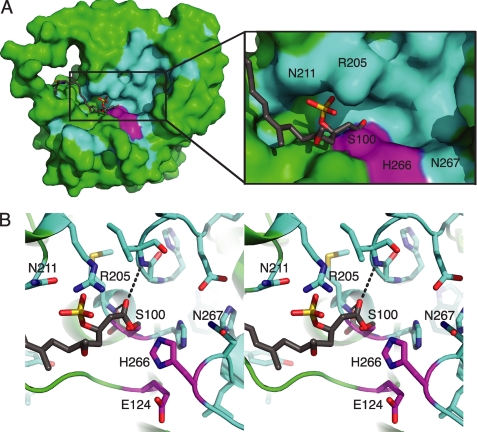
FIGURE 5.
CurM TE active site. A, surface of the active site cleft. In the molecular surface view, the active site (magenta catalytic triad) is in a cleft in which the presumed phosphopantetheine entrance is lined with conserved residues (cyan), with other parts of the protein surface in green and the modeled acyl-enzyme intermediate in gray sticks. B, modeled acyl-enzyme intermediate (gray C). In this stereo view, the intermediate is surrounded by conserved amino acid residues (cyan C) and the catalytic triad (Ser100, Glu124, and His266) (magenta C). The carbonyl oxygen is bound in the oxyanion hole (hydrogen bonds to the NHs of the Ile32 and Met101).