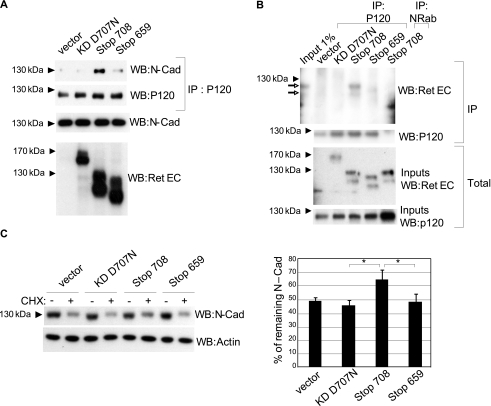
FIGURE 5.
Caspase-truncated RET interacts with P120 and increases N-cadherin stability. A, SH-SY5Y cells were transfected with different RET constructs and starved for 24 h. Formation of a P120-N-cadherin complex was analyzed by immunoprecipitation of P120 followed by N-cadherin Western blotting (WB). B, SH-SY5Y cells were transfected with different RET constructs and starved for 24 h. The formation of a complex between P120 and caspase-truncated RET was analyzed by immunoprecipitation of P120 or nonrelated antibody (NRab) followed by RET extracellular Western blotting. Open-headed arrow indicates the presence of mature and nonmature caspase-truncated RET. C, SH-SY5Y cells were transfected with different RET constructs. After 24 h, transfected cells were starved and exposed to cycloheximide (CHX) for 24 h. N-cadherin stability was quantified by Western blotting. Quantification corresponds to the average of three independent experiments. *, p < 0.05 analyzed by Student's test.