Abstract
There is a well-recognized need for low cost biodetection technologies for resource-poor settings with minimal medical infrastructure. Lab-on-a-chip (LOC) technology has the ability to perform biological assays in such settings. The aim of this work is to develop a low cost, high-throughput detection system for the analysis of 96 samples simultaneously outside the laboratory setting. To achieve this aim, several biosensing elements were combined: a syringe operated ELISA lab-on-a-chip (ELISA-LOC) which integrates fluid delivery system into a miniature 96-well plate; a simplified non-enzymatic reporter and detection approach using a gold nanoparticle-antibody conjugate as a secondary antibody and silver enhancement of the visual signal; and Carbon nanotubes (CNT) to increase primary antibody immobilization and improve assay sensitivity. Combined, these elements obviate the need for an ELISA washer, electrical power for operation and a sophisticated detector. We demonstrate the use of the device for detection of Staphylococcal enterotoxin B, a major foodborne toxin using three modes of detection, visual detection, CCD camera and document scanner. With visual detection or using a document scanner to measure the signal, the limit of detection (LOD) was 0.5ng/ml. In addition to visual detection, for precise quantitation of signal using densitometry and a CCD camera, the LOD was 0.1ng/ml for the CCD analysis and 0.5 ng/ml for the document scanner. The observed sensitivity is in the same range as laboratory-based ELISA testing. The point of care device can analyze 96 samples simultaneously, permitting high throughput diagnostics in the field and in resource poor areas without ready access to laboratory facilities or electricity.
Keywords: ELISA, Lab-on-a-chip, charge-coupled device, laser micromachining, microfluidics, Staphylococcal enterotoxins, silver enhancement, carbon nanotubes, point-of-care-testing, and resource-poor, food safety
1. Introduction
While the need for developing simple low cost Point of Care (POC) biodetection technologies for use in resource-poor settings with minimal medical infrastructure, such as in developing countries, is well recognized [1–3], most current biosensing and medical diagnostics technologies are relatively high cost devices designed for use in laboratories equipped with dedicated sophisticated equipment. The development of simple, low-cost, biosensing medical diagnostic technologies that can be used in the field has the potential to enhance healthcare delivery for the large fraction of the world’s population that lives in resource poor areas.
Currently lateral flow immunoassays [4] allow simple visual immunodetection in field settings, outside the laboratory. However, lateral flow assays have low sensitivity and are limited to a single sample for just a few analytes. Multi-sample or mult-analyte medical diagnostic immunodetection protocols still rely on laboratory-based detection methods, most commonly enzyme-linked immunosorbent assays (ELISA) [5],[6]. In ELISA, a primary antibody immobilized on solid surface is used to bind the antigen. A secondary labeled antibody is used to detect the captured antigen, and the binding of the secondary antibody is quantitated by measuring (in most cases optically) the activity of an enzyme bound to a secondary antibody. ELISA typically requires several reagent loading and washing steps, which are usually carried out by a dedicated washing device.
Traditional ELISA is enzyme-linked detection utilizing chromogenic reporters and substrates that produce detectable color changes to indicate the presence of antigen. More recently, fluorogenic and electrochemiluminescent enzymatic reporters and substrates have been developed. However, an the traditional enzyme-linked ELISA requires specialized reagents, including the enzyme and substrates and the chromogenic or flurogenic detection system, which creates a barrier for the application of the assay in resource poor areas. The reagents are relatively costly, have a limited shelf life, often require refrigeration, special storage conditions (for fluorogenic and electrochemiluminescent reagents) and can only be detected with the aid of sophisticated equipment (washer, detector). These factors limit the usefulness of conventional ELISA in outside the lab and in resource poor environments.
One goal of this work was to develop an alternative detection method that does not require an enzymatic reaction or specialized detector. Visual detection of gold labeling of the secondary antibody has been used an alternative to enzyme-labeled based immunodetection in lateral flow assays. However, this method is relatively less sensitive than conventional ELISA. Silver enhancement, a procedure in which gold nanoparticles act as nuclei for the silver aggregation, has been used to enhance detection. The silver enhancement occurs during the reduction of silver in the presence of gold particles and is rapid (within minutes). As a result, the silver forms aggregates on the surface of the gold particles, and the aggregates are visible as black deposits. The simplicity and high sensitivity of silver enhancement has been made it useful for light microscopy, electron microscopy, and blotting applications [7–10] but is not widely used in conventional ELISA. In this work, we incorporate a silver staining detection approach to an ELISA-LOC, eliminating the need for detection based on enzymatic activity and enabling visual detection. We have combined this detection method with a device described in previous work that incorporates a fluid delivery system into a miniature 96-well ELISA plate, eliminating the need for a washer [11]. The resulting ELISA-LOC with silver based immunological detection was tested in assays of Staphylococcal enterotoxin B (SEB) a common food toxin, with the aim of developing a visual detection Point of Care 96-well immunoassay
2. Experimental
2.1 Materials and Reagents
Reagents
Staphylococcal enterotoxin B (SEB) and rabbit anti-SEB affinity purified IgG were purchased from Toxin Technology (Sarasota, FL). Single-Walled Carbon Nanotube was obtained from Carbon Solutions Inc (Riverside, CA). Immun-Star HRP Enhanced chemiluminescence (ECL) kit was obtained from Bio-Rad (Hercutes, CA). Poly(diallyldimethylammonium chloride) polymer (PDDA), gold nanoparticles [G1527, ~0.75 A520 units/mL, 8.5–12.0 nm mean particle size (monodisperse), 10 nm particle size ], and silver enhancer kit (Catalog No: SE-100) were purchased from Sigma-Aldrich (St. Louis, MO). All other reagents were of analytical grade and de-ionized water was used throughout. The food used for the analysis (soy milk, apple juice and meat baby food) were purchased from a local store.
Materials for the fabrication of the 96-Well Sample Chips
clear 0.25 mm polycarbonate film and 1/8 inch black acrylic were obtained from Piedmont Plastics (Beltsville, MD).
Equipment
A Fisher (FS-14) Sonicator was obtained from Fisher Scientific (Pittsburgh, PA), and a Beckman mini-centrifuge was obtained from Beckman (Fullerton, CA).
2.2 preparation of the immunosensor
2.2.1 Carbon nanotube (CNT) preparation
The carbon nanotube (CNT) were prepared as previously described [12–14]. The CNTs (30 mg) were first shortened and functionalized by mixing with a concentrated sulfuric acid and nitric acid mixture (3:1 v/v) and sonicating with a Fisher (FS-14) sonicator for 6 h followed by extensive washing in water (100 ml) until neutralized (pH 7.0). Then the CNT were dispersed in 100 ml 1M NaOH solution for 5 min to achieve net negative charged carboxylic acid groups, and washed with water (100 ml). The positively charged polycation was adsorbed by dispersing the CNT in 50 ml of 1 mg/mL PDDA containing 0.5 M NaCl, for 30 min followed by centrifugation (10,000 RPM in Beckman centrifuge for 15 minutes) and washed with 100 ml of water.
2.2.2 CNT functionalization
The CNT were functionalized by dispersing in a rabbit anti-SEB IgG phosphate buffer solution (20 mM, pH 8.0) at a concentration of 0.01 mg/mL for 1 h at room temperature, so that the antibody was adsorbed onto the CNT surface. After centrifugation (15 minutes) and washing extensively with water (10 ml), the modified CNT were stored at 4°C in pH 8.0 phosphate buffer at a concentration of approximately 1mg/mL for no more than two weeks before use.
2.2.3 Preparation of gold nanoparticle-antibody conjugate
Antibody-gold nanoparticle conjugates was prepared as described [15] with minor modification. SEB antibody was added to 1 mL of pH-adjusted 10 nm gold nanoparticle solution with an antibody concentration of 0.01 mg/mL (pH 9.0 adjusted with 0.1 M K2CO3), followed by incubation at room temperature for 1 h. The conjugate was centrifuged at 14000 rpm for 30 min, and the soft sediment was resuspended in 1 mL of 0.01 M phosphate buffer (pH 7.4).
2.2.4 Fabrication and assembly of LOC
The LOC chips used for the immunosensor [11] were designed in CorelDraw11 (Corel Corp. Ontario, Canada) and micro-machined in 1/8 inch black acrylic using a computer controlled laser cutter Epilog Legend CO2 65W cutter (Epilog, Golden, CO). Before cutting, both sides of the acrylic sheet were coated with 3M 9770 adhesive transfer double sided tape (Piedmont Plastics, Beltsville, MD).
2.2.5. Immunosensor preparation
For preparation of the immunosensor, 15 μL of antibody modified CNT solution was dropped into the wells of 96 well chip, which were then dried and rinsed with washing buffer (20 mM phosphate buffer, pH 7.4) for 5 min to eliminate any loosely or partially immobilized CNT. The CNT-antibody modified wells were then blocked with 1% BSA for 30 min and incubated with different concentration of SEB in 15 μL of phosphate buffer for 45 min.
2.2.6 SEB LOC assay
After washing in 2 mL of phosphate buffer, the gold nanoparticle conjugated anti-rabbit IgG in buffer was loaded into LOC reaction wells by syringe and were incubated for 60 min at room temperature (25°C). The phosphate washing buffer was introduced to the wells the same way, and after washing 3 times, silver enhancement was carried out by adding enhancement buffer (a 1:1 volume ratio mixture of the two solutions from the silver enhancement kit) into each well and incubating in the dark for 10 min. The light intensity was then determined. In the presence of gold nanoparticles, the gold nanoparticles serve as nucleation sites to catalyze the reduction of silver ions to metallic silver, which changes the light intensity. Enhanced chemiluminescence (ECL) was achieved by adding ECL regent (formed by mixing the two solutions from the chemiluminescent kit in a 1:1 volume ratio) to the sample well. Since each 15 ul sample was loaded manually into a ~20 ul well, no sample-to-sample contamination was observed. For the 4-node design (which was not shown) each node is loaded independently along with the secondary antibody reagents so node to node contamination is avoided
2.4 Silver enhancement and ECL detection
For the CCD-based detection, a customized Point-of-care CCD detector [11–14, 16] was used. The CCD detector consists of an enclosed SXVF-M7 cooled CCD camera (Adirondack Video Astronomy, Hudson Falls, NY). The camera employs a Sony ICX-429ALL with 752x582 pixel CCD and is equipped with a 5mm extension tube and a 12mmPentax f1.2 lens (Spytown, Utopia, NY). Scanning based detection was done an office scanner, Savin 8055 from Ricoh, resolution 300 dpi, gray-scale mode (256 levels of gray). The CCD image intensities were analyzed using ImageJ software, developed and distributed freely by NIH (http://rsb.info.nih.gov/ij/download.html). The assay units are Signal to Noise Ratio (SNR), with each measured value divided by control (concentration 0 ng/ml)
3. Results and discussion
Several biosensing elements were combined to develop a simple, low-cost ELISA POC detection system: 1. a syringe operated lab-on-a-chip ELISA (ELISA-LOC), fabricated by lamination technology [11], which integrates fluid delivery system into a miniature 96 well ELISA plate. The ELISA-LOC does not need a dedicated washing instrument or electrical power for operation. 2. a gold nanoparticle-antibody conjugate secondary antibody in the sandwich assay, with amplification of the gold signal by silver enhancement into a visual signal, eliminating the need for a detector. 3. Carbon nanotubes (CNT) to increase primary antibody immobilization in the ELISA-LOC and thus improve assay sensitivity [12–14]. We compared three modes of detection for the ELISA-LOC, visual detection, CCD camera and document scanner for the detection of Staphylococcal enterotoxin B, a major foodborne toxin.
3.1 ELISA-LOC based assays
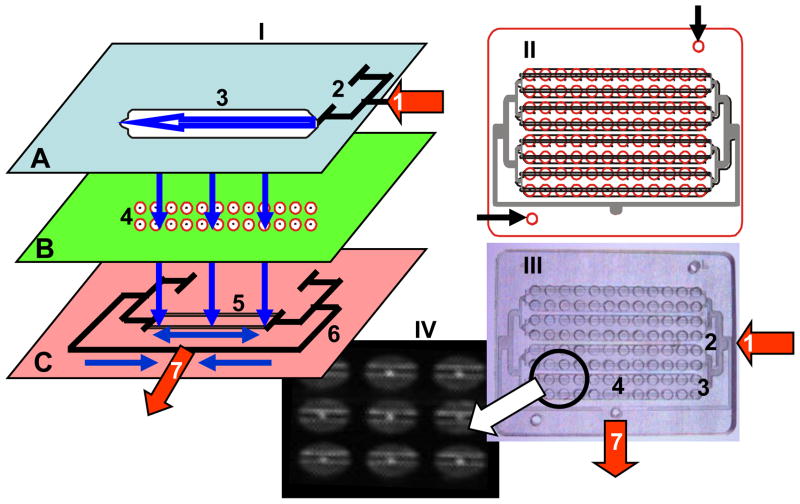
As shown in figure 1-I, the ELISA-LOC includes three major elements: 1) an interchangeable fluid delivery system (figure 1-I-A) which functions as the top of the plate and can be configured to perform assays in several different formats; 2) a miniature 96 well plate (figure 1-I-B) where the assays are carried out and detected; and 3) a fluid outlet system used to remove reagents from the wells (figure 1-I-C) attached to the bottom of the plate. The wells are needed to perform each of the 96 assays, after the initial binding of the antibody to the antigen in each well, all other steps (secondary antibody, washing etc) are shared between the wells. Figure 3I shows the device not the assay (shown in figure 2 and 3). Reagents enter through the inlet of the fluid delivery system (marked as arrow #1 in figure 1-A), distribute to four nodes via a distribution splitter (figure 1A # 2) and flow horizontally within the nodes of the fluid delivery system (shown as horizontal arrow marked #3 in figure 1A) and vertically between the layers’ fluid delivery system (shown as vertical arrows) to the 96 well plate (figure 1-I-B #4), and finally through holes in the wells into the outlet system (figure 1-I-C) with two outlet channels per node (Figure 1-I-C #5) placed directly under the well holes. The outlet channels are interconnected via two negative pressure distribution splitters (figure 1-I-C #6) connected to the outlet port (figure 1-I-C #7). The dimension of the microchip are 5.45x7.1 cm and the radius of the miniaturized microwell on chip is 1.65 mm and The thickness of channel is ~1–4 mm. The ELISA-LOC can be operated by a syringe (through the inlet #1 and outlet #7) so no external power is needed to operate the device. However, a peristaltic pump can be connected to the same outlets to simplify operations with the device.
Figure 1. ELISA-LOC.
I. An expanded 3D design of 96 well ELISA-LOC; II. The complete design overlay of the assembled 96 well device; III. Photo of the actual device; and IV. Fluorescein dye staining of the device showing the fine details of the wells. In the expanded diagram I, the three main layered elements of the LOC are: fluid delivery (A), which serves as the cover for the device, sample wells (B) for assay incubation and detection, and the outlet system (C) for fluid removal. For layer assembly, each layer is aligned using guide holes (marked with arrows in II) machined in all layers.
The main elements of ELISA-LOC are: fluid inlet port (1) connected to a pump or a syringe for reagent delivery, input fluid distribution splitter (2) which distributes reagents between the four nodes (a group of 24 wells; for the sake of simplicity, the figure only shows a single node in detail); and a fluid loading chamber (3) which is one of four chambers. The assay plate (B) includes the 96 wells for assay (4). Each well is fabricated with small holes in the bottom which serve as fluid outlets (shown as bright spots in IV). The outlet system (C) with two outlet channels per node (5) placed directly under the well holes. All the outlet channels are interconnected via negative pressure distribution splitters (6) connected to the outlet port (7) for a pump or syringe. The assembled schematic of the 96 well LOC chip (II), including the four nodes each with 2 rows of eight wells. A clear PMMA LOC device (III) was photographed with illumination to increase contrast and with fluorescein dye staining (IV) to show the fine details of the wells including the fluid removal holes and the outlet channels.
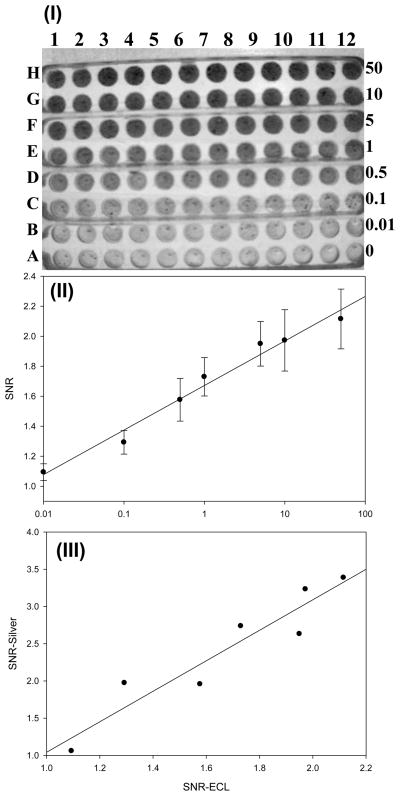
Figure 3. document scanner analysis of SEB detected with silver staining.
(I) document scanner image of the 96-well chip with different SEB concentrations. The binding was detected with gold-labelled secondary antibody, using silver enhancement. The wells contain, from bottom to top, 0, 0.01, 0.1,0.5,1,5,10, 50 ng/mL of SEB. Each concentration was measured 12 times (columns 1–12). (II) The corresponding plot of (I). Error bar = SD (n=12). The assay units are Signal to Noise Ratio (SNR), with each measured value divided by control (concentration 0 ng/ml)
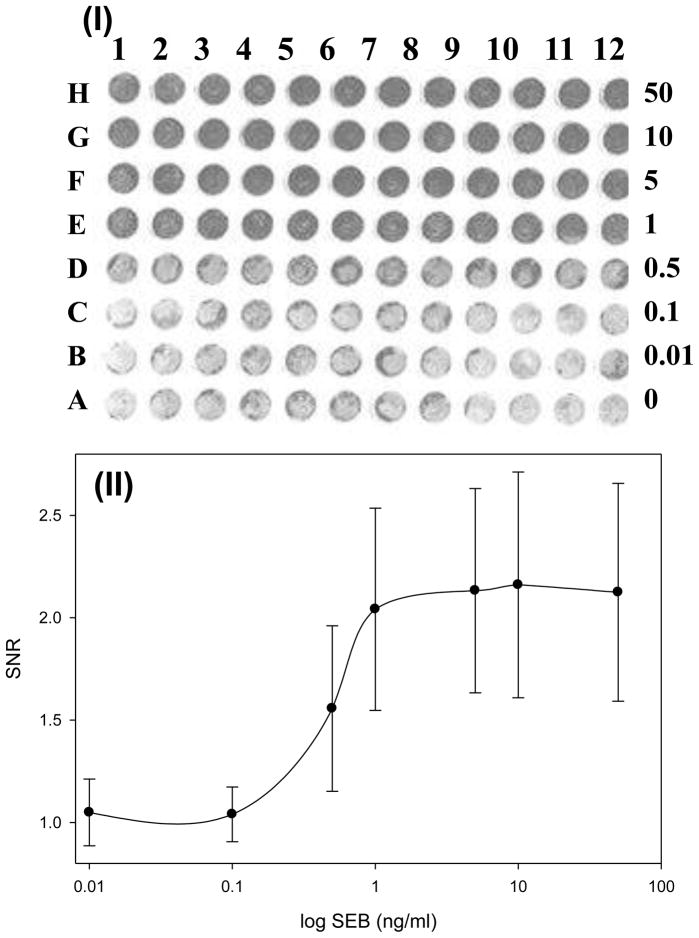
Figure 2. CCD analysis of SEB detected with silver staining.
(I) CCD image of the 96 chip with 12 replicas (rows) of 8 different SEB concentrations (columns) detected by silver enhancement detection. The SEB concentrations, from bottom (A) to top (H) are: 0, 0.01, 0.1,0.5,1,5,10, 50 ng/mL. Each concentration was measured 12 times (columns 1–12). (II) Signal intensities from the image were quantified using ImageJ and the dose response curve was plotted. The assay units are Signal to Noise Ratio (SNR), with each measured value divided by control (concentration 0 ng/ml). The error bar = SD (n=12). (III) Comparison between ELISA-LOC detected by silver staining and Enhanced Chemiluminescence detection. The silver staining results for SEB (II) were very similar to results from a similar chip detected with ECL
The design overlay of the ELISA-LOC (figure 1-II) and pictures of the actual device (figure 1-III) show its major features. To visualize the details, the device was loaded with fluorescein dye and photographed, after washing (figure 1-IV). The wells with the outlet holes (the bright spots in the center of the wells) and the outlet channels (the bright lines across the wells) are shown clearly in this image. The fluids are kept in the wells by surface tension. A simple ‘surface tension’ valve was created by drilling a ~0.2 mm hole in the bottom of the well. When a vacuum is applied at the outlet (#7), fluid flows through the holes and empties the wells into the outlet channels.
3.2 ELISA-LOC analysis of SEB detected with silver staining
In ELISA-LOC detection of SEB, anti-SEB primary antibody was conjugated onto a carbon nanotube (CNT) surface that was immobilized into each well during ELISA-LOC fabrication. We have shown previously [12–14] that the large surface area of CNTs increase the sensitivity of immunoassays approximately six-fold. A serial dilution of SEB samples (15 ul) at a range of concentrations (0.01–50 ng/mL) and a control (no SEB) were loaded manually into the wells of the plate (figure 2-I, columns A-H), in 12 replicas (rows 1–12). After adding the SEB samples into the antibody-CNT wells, the cover containing the fluid delivery system (figure 1-I-A) was bonded to the assay plate (using the double sited tape bonded to the fluid delivery system during fabrication) and the ELISA-LOC was incubated for 60 minutes.
For this assay, all reagents were loaded sequentially into the loading fluid delivery system inlet (figure 1, I-A #1) using the outlet shown in figure 1, I-C #1. Because the same reagents (washing buffer, secondary antibody, silver reagents) are used for all samples, cross mixing and diffusion among channels were not factors. After a one hour incubation at room temperature the plate was washed with 2 ml of phosphate buffer delivered with syringe via the fluid delivery system. The buffer was removed via the fluid outlet system, which was bonded to the bottom of the plate. Following washing, the binding of SEB to the primary antibody was detected using 2 ml of gold labeled secondary antibody which was injected via the fluid delivery system and incubated for 1 h. This was followed by 3 more cycles of washing with 2 ml of phosphate buffer as described above. The gold imaging was enhanced by silver staining. The silver enhancement allowed the signal to be visible by eye. In addition, the image was captured by a CCD camera and analyzed and quantitated by imageJ.
Visual detection of ELISA-LOC
A CCD image of a representative assay is shown in figure 2-I. Visual inspection suggests that the there is a difference between the SEB signal in row D (0.5 ng/ml) and the no-SEB control in row A. Regarding visual detection, it is a subjective measurement similar to lateral flow assay strips. It is not very quantitative. Based on the observed results, the limit of visual detection of the ELISA-LOC with silver enhancement is ~0.5 ng/ml of SEB.
Quantitation of signals from silver stained ELISA-LOC
In addition to the visual analysis, signal intensities from the CCD camera (figure 2-I) were quantified and the dose response curve was plotted. The CCD image intensities were analyzed using ImageJ software as described in the Materials & Methods. The assay units are Signal to Noise Ratio (SNR), with each measured value divided by control (concentration 0 ng/ml). The image intensities show an increase in the signal corresponding to increasing concentration of SEB (figure 2-II). A statistical analysis shows very high correlation (r2=0.98) between SEB concentration and density signals. The limit of detection (LOD) represents a measured concentration that generates a signal three times the standard deviation above background (no SEB). For SEB measured with the ELISA-LOC and detected using a CCD camera, the LOD is 0.1 ng/ml (row C).
Comparison between ELISA-LOC detected by silver staining and Enhanced Chemiluminescence (ECL) detection
We compared SEB detected by gold nanoparticle-silver enhancement with a method using Enhanced Chemiluminescence (ECL) for the detection of by HRP labeled antibody. The ECL assay was performed in a similar manner, instead gold labeled secondary antibody HRP labled antibody was used followed by washing with 2 ml of ECL buffer (mixing the two solutions from Chemiluminescent Kit in a 1:1 volume ratio) and measuring ECL intensity by CCD. In the presence of HRP (bound to the secondary antibody), luminol in the ECL reagents reduces hydrogen peroxide, which emits light as it returns to its basal state, and is detectable using a CCD.
The same camera was used to capture both images. As shown in figure 2-III, the methods are very similar in terms of the level of detection and linearity. There is a high correlation (r2=0.89) between the silver staining results for SEB to the results from a similar chip detected with ECL. As with silver-enhanced detection, the limit of detection for ECL is also 0.1 ng/ml, suggesting that both detection methods are comparable. For the standard ELISA assays for Staphylococcal enterotoxins, the reported LOD ranges from 0.1–10 ng/mL [17].
Document scanner analysis of ELISA-LOC
Commercial desktop document scanners have been used for densitometry analysis of biological measurements [18–23], so we tested one in the black and white document mode for detection of the ELISA-LOC signals. The scanned image is shown in figure 3-I. Signal intensities from the scanner (figure 3-I) were quantified using ImageJ and the dose response curve was plotted (figure 3-II). The limit of detection using a scanner is 0.5 ng ml. However, the standard deviation (SD) of these measurements is relatively large and dynamic range of the image in this 8 bits scanner is narrow (0.1–1 ng/ml) compared to CCD imaging (figure 2-I). The main advantage of this type of limited dynamic range scanning is that it provides a simple YES/NO result with a narrow cut off, similar to the results from analysis by lateral flow detection. For many situations, such as rapid testing for the presence of a toxin or pathogen, or in situations where data interpretation is carried out by untrained users, such YES/NO type analysis may simplify diagnostics.
4. Conclusion
The ELISA-LOC integrates a washing system onto an ELISA plate, allowing 96 well analysis outside of a lab. The combination of ELISA-LOC with silver enhancement of staining produced a signal that was visually detectable. Thus, this system represents a simple assay that can be used even in resource-poor settings without laboratory equipment. We used a cooled CCD described in Material and Methods simply because we had the camera. However since we detect change in black and white density, any low cost camera can be used
As shown, the results of ELISA-LOC can be detected visually for SEB concentrations as low as 0.5 ng/ml. Visual detection is semiquantitative but is a very low cost approach for biodetection. Using a document scanner had the same LOD (0.5 ng/ml), and provided the user with a simple yes/no answer. Use of a CCD camera for detection improves the sensitivity to 0.1 ng/ml.
Gold-silver detection is a good replacement for the commonly-used enzymatic assays, because it eliminates the complexity of enzyme-based detection while retaining the sensitivity of the enzymatic based detection.
Our approach for point of care testing may overcome the limitations of the traditional enzyme-linked ELISA including the need for specialized reagents (enzyme and substrates and the chromogenic or flurogenic detection system) which are relatively costly, have a limited shelf life and often require refrigeration, special storage conditions. The shelf life: gold and silver reagents and CNTs are stable at room temperature and normally do not requires special storage condition, even for long-term storage In addition we eliminated the need for specialized equipment (washer, detector) which requires power for operation. These factors limit the usefulness of conventional ELISA in outside the lab and in resource poor environments. In term of assay costs, for the ECL detection, the cost of reagents (primary and secondary antibodies+ ECL reagents) for SEB detection using a regular ELISA plate (e.g. 100ul microtiter plate) is ~$1 per assay. Using our 20ul miniature plate for the same ECL assay the price per assay is ~20 cents per assay. For the gold nanoparticles assay (primary and secondary antibodies + gold nanoparticles and silver enhancement reagents) the price is ~80 cents per 100ul ELISA assay and ~16 cents for the minature plate assay. So the new assay is significantly cheaper, both because a lower volume of reagent is used and the cost of the actual reagents is lower. Moreover, the instrumentation cost for the assay has been reduced dramatically, replacing costly (e.g. $3,000-$20,000) plate reader with ~$100 CCD camera or scanner and eliminating the need for a washer (~$2500–$10,000).
The current ELISA-LOC was designed to be disposable and to reduce cost. While the interchangeable fluid delivery system (figure 1-I-A) and the fluid outlet system used to remove reagents from the wells (figure 1-I-C) are both reusable, they are both readily manufactured and low-cost enough to be disposable as well. : As a prototype the device may look complicated. However it is relatively simple to mass produce (by simply assembling three layers). However the current system is not automated which complicate the use of ELISA-LOC, low cost automation (underdevelopment) may simplify the use of the system.
The advantage of using a document scanner or a CCD camera is that it enables the quantitation and transmission of the assay data to other locations and thus increases the versatility of the system, even though it adds to the cost of the system. As shown in previous work [12–14], partial purification and concentration of the SEB in a sample allows detection by ECL at levels as low as 0.01 ng/ml[12]. However, such purification and concentration steps require more complex protocols and equipment, which would not be available in the field settings where the ELISA-LOC is intended for use. The ELISA-LOC has several features intended to make it a portable and suitable for field settings, where no laboratory is available. These include operation by a syringe, so no external or battery power is required, and capacity to analyze 96 samples simultaneously without a lab, which significantly improve point of care detection capabilities. While much of current biosensing and medical diagnostics technologies are costly and designed to be performed in laboratories equipped with dedicated lab equipment, these are not always available in poor regions of the world. The approach described here has the potential to address the detection and diagnostics needs of medically underserved and resource poor areas.
Biographies
Steven Sun studied at the University of Maryland Baltimore County (UMBC) and received a BS in computer engineering in 2007. He continued his education at UMBC and is currently pursuing a MS in computer engineering with a focus in optics and chip design, and biosensors. In 2007 he began work as a research engineer at the US Food and Drug Administration (FDA) in the Office of Science and Engineering Laboratories (OSEL). His work involves biotechnology and applications of computer engineering in the biosensor field for public health safety.
Yordan Kostov received his B.Sc. and M.Sc. with honors in 1987 from Department of Electrical Engineering, Odessa Politechnic Institute, Former USSR. He received a Ph.D. Degree in Electrical/Chemical Engineering from Bulgarian Academy of Sciences. From 1994 to 1999 he was an Assistant Professor in the Department of Biotechincs, Sofia Technical University, Bulgaria. After post-doctoral fellowship at Medical Biotechnology Center, University of Maryland Biotechnology Institute, in 2000 he became Research Assistant Professor at the Department of Chemical and Biochemical Engineering, University of Maryland, Baltimore County (UMBC). Since 2006 he is a Research Associate Professor at the same department, and he also holds a position as an Assistant Director of the Center for Advanced Sensor technology at the same University. He is involved in the development of chemical and biochemical sensor systems for biotechnology and biomedical applications.
Minghui Yang is a professor of chemistry at the University of Jinan (China), conducting research on nanomaterial based biosensors. Dr. Yang obtained a BSc in Chemical Engineering from the Hunan Nanhua University in 2001 and a PhD from Hunan University Dept. of Analytical chemistry in 2007 for research on Chemistry and fabrication of nanomaterial. From 2007 to 2009, he conducted research at the Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) conducting research on the application of nanomaterial to biosensing where he developed the concept of Electrical percolation biological semiconductor. Dr. Yang is continuing his research on biosensors at the University of Wisconsin-Milwaukee (UWM).
Avraham Rasooly obtained a BSc in Agronomy from the Hebrew University Faculty of Agriculture in 1978, an MSc in Genetics from the Hebrew University Department of Genetics, in 1982 and a PhD from the Michigan State University Dept. of Crop & Soil Science in 1988 for research on microbial nitrogen fixation. From 1988 to 1990, he conducted postdoctoral research first at Michigan State University on the genetics of fungal lignin degradation, and then from 1990 to 1995 as a Research Assistant Professor/Senior Research Scientist in the Dept. of Molecular Pathogenesis, Skirball Institute NYU Medical Center and the Public Health Research Institute of New York working on microbial genetics, the regulation of plasmid replication and S. aureus pathogenesis. Dr. Rasooly joined the Food and Drug Administration (FDA) as a Microbiologist in 1995, where he is developing microbial detection approaches for foodborne pathogens and their toxins using biosensors and DNA microarrays. Currently Dr. Rasooly holds a joint FDA Center for Devices and Radiological Health (CDRH) and National Cancer Institute (NCI) appointment, continuing his research on microbial detection at FDA and serving as a program director at the Cancer Diagnostics Program of NCI, where he oversees research on novel technologies of cancer diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hay Burgess DC, Wasserman J, Dahl CA. Global health diagnostics. Nature. 2006;444(Suppl 1):1–2. doi: 10.1038/nature05440. [DOI] [PubMed] [Google Scholar]
- 2.Urdea M, Penny LA, Olmsted SS, Giovanni MY, Kaspar P, Shepherd A, Wilson P, Dahl CA, Buchsbaum S, Moeller G, Hay Burgess DC. Requirements for high impact diagnostics in the developing world. Nature. 2006;444(Suppl 1):73–79. doi: 10.1038/nature05448. [DOI] [PubMed] [Google Scholar]
- 3.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annual review of biomedical engineering. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 4.van Amerongen A, Wichers JH, Berendsen LBJM, Timmermans AJM, Keizer GD, van Doorn AWJ, Bantjes A, van Gelder WMJ. Colloidal carbon particles as a new label for rapid immunochemical test methods: Quantitative computer image analysis of results. Journal of Biotechnology. 1993;30:185–195. doi: 10.1016/0168-1656(93)90112-z. [DOI] [PubMed] [Google Scholar]
- 5.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 6.Van Weemen BK, Schuurs AH. Immunoassay using antigen-enzyme conjugates. FEBS letters. 1971;15:232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- 7.Tang J, Xu Z, Zhou L, Qin H, Wang Y, Wang H. Rapid and simultaneous detection of Ureaplasma parvum and Chlamydia trachomatis antibodies based on visual protein microarray using gold nanoparticles and silver enhancement. Diagn Microbiol Infect Dis. 2010;67:122–128. doi: 10.1016/j.diagmicrobio.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Liu Q, Li Z, Zhong W, He W, Xu D. A visual chip-based coimmunoprecipitation technique for analysis of protein-protein interactions. Analytical biochemistry. 2010 doi: 10.1016/j.ab.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Yang M, Wang C. Label-free immunosensor based on gold nanoparticle silver enhancement. Analytical biochemistry. 2009;385:128–131. doi: 10.1016/j.ab.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Girigoswami A, Li T, Jung C, Mun HY, Park HG. Gold nanoparticle-based label-free detection of BRCA1 mutations utilizing DNA ligation on DNA microarray. Journal of nanoscience and nanotechnology. 2009;9:1019–1024. doi: 10.1166/jnn.2009.c077. [DOI] [PubMed] [Google Scholar]
- 11.Sun S, Yang M, Kostov Y, Rasooly A. ELISA-LOC: lab-on-a-chip for enzyme-linked immunodetection. Lab on a chip. 2010 doi: 10.1039/c003994b. [DOI] [PubMed] [Google Scholar]
- 12.Yang M, Kostov Y, Bruck HA, Rasooly A. Carbon nanotubes with enhanced chemiluminescence immunoassay for CCD-based detection of Staphylococcal enterotoxin B in food. Analytical chemistry. 2008;80:8532–8537. doi: 10.1021/ac801418n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang M, Kostov Y, Rasooly A. Carbon nanotubes based optical immunodetection of Staphylococcal Enterotoxin B (SEB) in food. International journal of food microbiology. 2008;127:78–83. doi: 10.1016/j.ijfoodmicro.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Yang M, Kostov Y, Bruck HA, Rasooly A. Gold nanoparticle-based enhanced chemiluminescence immunosensor for detection of Staphylococcal Enterotoxin B (SEB) in food. International journal of food microbiology. 2009;133:265–271. doi: 10.1016/j.ijfoodmicro.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan A, Lau C, Lu J. Magnetic bead-based chemiluminescent metal immunoassay with a colloidal gold label. Analytical chemistry. 2005;77:3238–3242. doi: 10.1021/ac050163b. [DOI] [PubMed] [Google Scholar]
- 16.Yang M, Sun S, Kostov Y, Rasooly A. Lab-on-a-chip for carbon nanotubes based immunoassay detection of Staphylococcal Enterotoxin B (SEB) Lab on a chip. 2010;10:1011–1017. doi: 10.1039/b923996k. [DOI] [PubMed] [Google Scholar]
- 17.Bennett RW. Staphylococcal enterotoxin and its rapid identification in foods by enzyme-linked immunosorbent assay-based methodology. J Food Prot. 2005;68:1264–1270. doi: 10.4315/0362-028x-68.6.1264. [DOI] [PubMed] [Google Scholar]
- 18.Clarke ML, Burton RL, Hill AN, Litorja M, Nahm MH, Hwang J. Low-cost, high-throughput, automated counting of bacterial colonies. Cytometry A. 2010 doi: 10.1002/cyto.a.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo HI, Kim J, Cho NI. Composition of a dewarped and enhanced document image from two view images. IEEE Trans Image Process. 2009;18:1551–1562. doi: 10.1109/TIP.2009.2019301. [DOI] [PubMed] [Google Scholar]
- 20.Stevens DY, Petri CR, Osborn JL, Spicar-Mihalic P, McKenzie KG, Yager P. Enabling a microfluidic immunoassay for the developing world by integration of on-card dry reagent storage. Lab on a chip. 2008;8:2038–2045. doi: 10.1039/b811158h. [DOI] [PubMed] [Google Scholar]
- 21.Oakham P, MacDougall RD, Rowlands JA. The optimal optical readout for the x-ray light valve--document scanners. Med Phys. 2008;35:5672–5683. doi: 10.1118/1.3006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalef-Ezra J, Karava K. Radiochromic film dosimetry: reflection vs transmission scanning. Med Phys. 2008;35:2308–2311. doi: 10.1118/1.2919092. [DOI] [PubMed] [Google Scholar]
- 23.Devic S, Wang YZ, Tomic N, Podgorsak EB. Sensitivity of linear CCD array based film scanners used for film dosimetry. Med Phys. 2006;33:3993–3996. doi: 10.1118/1.2357836. [DOI] [PubMed] [Google Scholar]