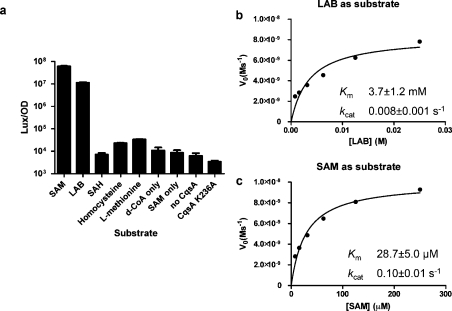
Figure 2.
Potential substrates for CqsA and kinetic studies of SAM and LAB as CqsA substrates. (a) Different compounds were examined for activity as CqsA substrates. Reactions were carried out with each potential substrate at 1 mM and d-CoA at 100 μM. Control reactions with SAM only (no d-CoA), without CqsA, and with an inactive CqsA mutant CqsA K236A(13) are also shown. (b, c) Coenzyme A release was measured by a coupled enzyme assay. Kinetic constants for LAB (b) and SAM (c) were estimated by fitting initial velocities to the Michaelis−Menten equation using GraphPad Software.