Abstract
A 16-year-old Paint stallion was presented with intermittent fever, inappetance, lethargy, icterus, distal limb edema, and submandibular lymphadenopathy. The horse was native to Nova Scotia and had never left that province. Morulae were detected in granulocytes. Ananaplasma phagocytophilum infection was confirmed by serology and polymerase chain reaction (PCR). The horse responded to treatment with oxytetracycline.
Résumé
Anaplasmose granulocytaire chez un cheval de la Nouvelle-Écosse causée par une infection par Anaplasma phagocytophilum. Un étalon Paint âgé de 16 ans a été présenté avec une fièvre intermittente, de l’inappétence, de l’abattement, de l’ictère, un œdème du membre distal et une lymphadénopathie sous-maxillaire. Le cheval était originaire de la Nouvelle-Écosse et n’avait jamais quitté la province. Des morulas ont été détectées dans les granulocytes. L’infection par Ananaplasma phagocytophilum a été confirmée par sérologie et une réaction d’amplification en chaîne par la polymérase (PCR). Le cheval a répondu au traitement à l’oxytétracycline.
(Traduit par Isabelle Vallières)
A 16-year-old, 410-kg, Paint stallion from Nova Scotia was referred to the Atlantic Veterinary College (AVC) Teaching Hospital in early December, 2009 with a 5-day history of depression, partial anorexia, and intermittent fever spikes in the evening, ranging from 38.8°C to 39.8°C. Two days prior to presentation, the horse was treated once with procaine penicillin G (source unknown) at 9 000 000 IU, IM, followed by 4 500 000 IU, IM, q12h. There was no improvement in clinical signs. The horse had distal limb edema 1 day before referral and developed a mild cough on the second day of illness. The cough resolved in response to feeding hay that had been soaked in water.
The stallion was turned out alone on a designated pasture and was separated from other horses on the premises by a 3-m gap between pasture fence lines and a 3-m high wall in the barn. The horse had never left Nova Scotia, but had competed in penning competitions throughout the province. Vaccination history for the previous spring was uncertain but, prior to that time, vaccinations against equine influenza, equine rhinopneumonitis, tetanus, strangles, and West Nile virus had been given annually. An ivermectin dewormer had been administered 1 mo prior to presentation. The horse had never been tested for antibodies to equine infectious anemia virus.
Upon presentation to the AVC, the stallion was quiet but responsive with a rectal temperature of 38.1°C, heart rate of 40 beats/min, and respiratory rate of 32 breaths/min. Breath sounds and expiratory effort were increased, but no adventitious sounds were heard upon application of a rebreathing bag. Mucous membranes were pink and moist with a capillary refill time of < 2 s. The sclerae were moderately injected and icteric, and mild bilateral submandibular lymphadenopathy was noted. Edema was present in the distal half of all 4 limbs and also in the ventral abdominal and pectoral regions.
Differential diagnoses for the intermittent fever, icterus, limb and ventral edema, scleral injection, and submandibular lymphadenopathy exhibited by the patient included infectious diseases (equine viral arteritis, equine infectious anemia, equine granulocytic anaplasmosis) and immunologic diseases (purpura hemorrhagica associated with Streptococcus equi subsp. equi and other immune-mediated vasculitides). Initial diagnostic tests included a complete blood (cell) count (CBC) and biochemistry profile.
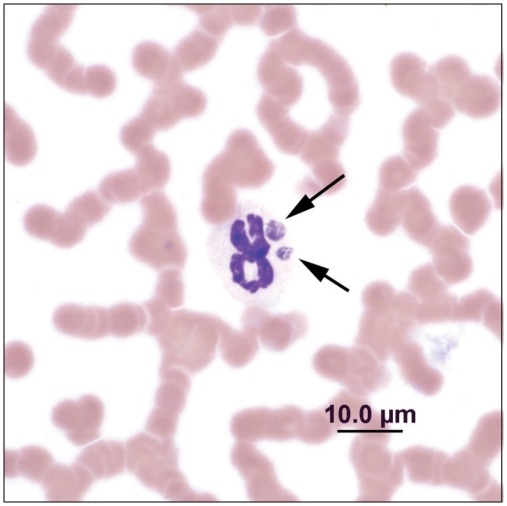
Hematology analysis using a Sysmex XT 2000iV analyzer (Sysmex Corporation, Kobe, Japan) revealed a slight normocytic, normochromic anemia [0.31 L/L; reference interval (RI): 0.32 to 0.52 L/L]. While this mild change could reflect patient variation, decreased erythrocyte production, hemorrhage, or hemolysis were possible. The neutrophil count was within reference limits (5.33 × 109/L; RI: 2.70 to 6.70 × 109/L) with a marginal left shift (0.14 × 109/L; RI: 0.00 to 0.10 × 109/L) and a mild lymphopenia (1.35 × 109/L; RI: 1.50 to 5.50 × 109/L). These findings supported very mild acute inflammation coupled with a stress response. A moderate thrombocytopenia (estimated to be 75 to 100 × 109/L; RI: 310 to 510 × 109/L; clumped platelets precluded an accurate automated platelet count) was also present. Considerations for the thrombocytopenia included increased utilization, decreased production, increased destruction and increased sequestration. Review of the blood smear revealed single to rarely 2, round to ovoid, 2- to 4-μm diameter, blue, granular morula-like cytoplasmic inclusions within approximately 16% of neutrophils (32 per 200 neutrophils throughout the monolayer; Wright-Giemsa stain, Ames Hematek-1000, Bayer Corporation, Elkhart, Indiana, USA) (Figure 1).
Figure 1.
Two Anaplasma phagocytophilum morulae (arrows) in a neutrophil. Blood smear, 100×, Wright’s-Giemsa stain.
Serum biochemistry analysis (Cobas 6000 c501 analyzer; Roche Diagnostics, Basel, Switzerland) indicated minor, non-specific electrolyte and biochemical changes including a mild hyponatremia (133 mmol/L; RI: 135 to 148 mmol/L), hypochloremia (97 mmol/L; RI: 98 to 110 mmol/L), hypokalemia (2.7 mmol/L; RI: 3.0 to 5.0 mmol/L), hypophosphatemia (0.89 mmol/L; RI: 1.0 to 1.8 mmol/L), hypomagnesemia (0.69 mmol/L; RI: 0.74 to 1.02 mmol/L), hyperglycemia (6.9 mmol/L; RI: 3.6 to 5.6 mmol/L), and hypoalbuminemia (23 g/L; RI: 25 to 36 g/L).
Based on the finding of morulae in the neutrophils of the horse, a presumptive diagnosis of equine granulocytic anaplasmosis was made. The stallion was carefully inspected for the presence of ticks but none were found. To further substantiate a diagnosis of anaplasmosis, whole blood and serum samples obtained at admission were submitted for polymerase chain reaction (PCR) (Lucy Whittier Molecular and Diagnostic Core Facility — Taq/Man Service, Department of Medicine and Epidemiology, School of Veterinary Medicine Davis, California, USA) and serologic testing (Diagnostic Centre for Population and Animal Health, Michigan State University, Lansing, Michigan, USA) for Anaplasma phagocytophilum. The horse was PCR positive for A. phagocytophilum and had an indirect fluorescent antibody (IFA) titer of 10 240. A titer of > 160 is considered positive for A. phagocytophilum.
Treatment was initiated with oxytetracycline (Vétoquinol, Lavaltrie, Quebec) diluted in 250 mL of saline at 7 mg/kg, IV, q24h for 7 d and flunixin meglumine (Vétoquinol) at 0.5 mg/kg, IV, once, on the day of admission when the horse developed a fever of 39.7°C. Leg wraps were applied to all 4 limbs and the hay was soaked in water prior to being fed. After the first day of hospitalization the horse remained afebrile. Signs of icterus, scleral injection, lymphadenopathy, and limb edema gradually resolved and were absent 6 d after initiation of treatment with oxytetracycline. However, because the stallion continued to have a poor appetite, gastric ulceration was suspected and treatment was initiated with sucralfate (Novopharm, Toronto, Ontario) at 20 mg/kg, PO, q8h on the third day of hospitalization and continued until the patient was discharged with an improved appetite 7 d after being admitted.
A CBC was repeated on day 4 of treatment. The leukon was within reference limits. A slight normocytic, normochromic anemia (0.30 L/L; RI: 0.32 to 0.52 L/L) was present, similar to the erythron findings on admission. There was a mild increase in fibrinogen concentration (9 g/L; RI: < 5 g/L). This likely reflected an acute phase inflammatory response as current mild hyperproteinemia (80 g/L; RI: 60 to 77 g/L), moderate hyperglobulinemia (58 g/L; 35 to 41 g/L) and mild hypoalbuminemia (22 g/L; RI: 25 to 36 g/L) with a low A:G ratio (0.38; RI: 0.60 to 1.50) were also present. Hemoconcentration leading to hyperfibrinogenemia was unlikely given the low A:G ratio. Clumped platelets precluded an accurate automated platelet count but platelets appeared adequate in numbers based on blood smear estimation. No individual Anaplasma species organisms or morulae were observed. On day 7, the last day of treatment, a CBC revealed a slight normocytic, normochromic anemia with identical parameters to day 4. The fibrinogen concentration and platelet counts were within reference limits and no individual organisms or morulae were observed in neutrophils. The horse was discharged 8 d after admission and has remained clinically healthy.
Anaplasma phagocytophilum, first described as the cause of equine granulocytic ehrlichiosis in horses in 1969, is an obligate intracellular, gram-negative coccoid bacterium (1,2). The bacterium has a tropism for granulocytes and is most commonly found in neutrophils as cytoplasmic inclusions (morulae) (1). Organisms are found in membrane-lined vacuoles and stain pale blue-gray to deep blue with Wright-Giemsa staining (2).
In 2001, 3 species of granulocytic bacteria causing disease in horses (Ehrlichia equi), ruminants (Ehrlichia phagocytophilum) and humans (human granulocytic ehrlichiosis agent) were designated as variants of the same species, Anaplasma phagocytophilum, to reflect the close genome homology and similarity in pathophysiology (3). To remain consistent with the current nomenclature, the term equine granulocytic anaplasmosis (EGA) is used in this report to refer to the clinical syndrome produced by Anaplasma phagocytophilum in horses (1).
The bacterium is transmitted most notably by ticks of the complex Ixodes ricinus, with regional species differences (1). Ixodes pacificus and I. scapularis are the primary tick species involved in transmission in western and eastern North America, respectively (1). Wildlife can act as a reservoir host for A. phagocytophilum and domestic animals and humans become infected through tick bites. Birds are increasingly implicated as potential reservoirs and migratory birds may disperse ticks, expanding the range of tick populations into new locations (1,4).
Horses with EGA may present with clinical signs varying in severity from asymptomatic to life-threatening. Death due to EGA is exceedingly rare; only 1 report of death as a direct consequence of the disease has been found (5). Death due to secondary infections or following injury due to incoordination has been reported (6). Clinical signs most commonly occur in fall, winter, and spring (7,8). The horse in this report presented in December, which is consistent with the seasonal findings of EGA. Clinical signs are due to the elicited inflammatory response as well as a necrotizing vasculitis resulting in fever, anorexia, lethargy, limb edema, petechiae, icterus, reluctance to move, and ataxia (8). Signs appear to be more severe in older horses (7,8). Although EGA is self-limiting in 2 to 3 wk, treatment with tetracycline antibiotics is effective and shortens the disease course significantly, provided there are no secondary complications (7,8).
Characteristic hematological abnormalities in horses infected with A. phagocytophilum include anemia, leucopenia, and thrombocytopenia (8). The leucopenia is often characterized by neutropenia, particularly during the febrile stage of infection. Thrombocytopenia is a frequent finding in many Anaplasmataceae infections (7,9,10). The exact mechanism of thrombocytopenia is unknown, but previous work in dogs suggests thrombocytopenia may be due to platelet destruction (9).
Protective immunity develops after natural and experimental infection, but the duration appears variable. Antibodies were detected in horses up to 12 mo after natural infection and experimental inoculation resulted in immunity against reinfection for up to 20 mo (2,11). A carrier state does not develop and prevention is currently primarily focused on tick control.
A diagnosis of EGA can be made when characteristic intra-granulocytic morulae are seen on a blood smear (8). Although visualization of morulae is specific for the diagnosis of EGA, this diagnostic tool has a limited sensitivity because morulae are present for only a short time period during the disease (12). Morulae are primarily observed during the early phase of the disease, when bacteremia peaks, and are typically absent in later stages of infection (1). Absence of morulae during the first few days of fever has also been reported (12). Taken together, these reports highlight the possibility of false negative results if diagnosis is based solely on detection of morulae. Morulae can be found in up to 40% of circulating neutrophils at the peak of bacteremia (7,8,11,13). In contrast, in ruminant species, up to 90% of granulocytes may be infected during the peak period of bacteremia, which also tends to last longer than in the horse (1). The horse in this report had approximately 16% morula-positive neutrophils. A low percentage of morula-positive neutrophils in this horse may have been due to the stage of infection or patient or strain variation. A PCR assay is commercially available and allows rapid and sensitive, early diagnosis, especially in the absence of observable morulae. A convalescent 4-fold serum titer increase also confirms the diagnosis and response to treatment with oxytetracycline, but not other antibiotics, is supportive of a diagnosis of EGA. The horse in this report had intraneutrophilic morulae that facilitated a diagnosis of A. phagocytophilum. Serologic testing and PCR were performed after detection of morulae to verify A. phagocytophilum infection. Had the morulae not been observed, and because EGA had not previously been reported in Atlantic Canada, it is questionable whether further diagnostic steps would have been taken to specifically identify A. phagocytophilum. As such, this case highlights the importance of routine blood smear evaluation.
To the authors’ knowledge, this is the first report of EGA in Atlantic Canada and only the second report of this disease in a horse in Canada (13). Equine granulocytic anaplasmosis has been reported in certain regions of the USA, including northern California, New England, and the mid-Atlantic and upper Midwestern states (7). It has also been diagnosed in Central America and a variety of European countries (7). In Canada, A. phagocytophilum infections of veterinary importance have been reported in 1 dog and 1 horse from Vancouver Island and 3 dogs from Saskatchewan (10,13,14). A single case of A. phagocytophilum infection in a dog traveling from Toronto to Prince Edward Island has also been seen at the AVC (Clancey, personal observation). The diagnosis of EGA in a horse permanently residing in Nova Scotia may indicate expansion of the geographical presence of A. phagocytophilum-positive I. scapularis and should increase awareness of possible emergence of this disease in Atlantic Canada. It is uncommon to test horses from Atlantic Canada for A. phagocytophilum as it has not been reported previously. However, it may now be prudent to request PCR and/or serologic testing in horses from Atlantic Canada that exhibit clinical signs compatible with EGA, especially when characteristic morulae are not observed on blood smear evaluation. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet Parasitol. 2010;167:108–122. doi: 10.1016/j.vetpar.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Gribble DH. Equine ehrlichiosis. J Am Vet Med Assoc. 1969;155:462–469. [PubMed] [Google Scholar]
- 3.Dumler JS, Barbet AF, Bekker CPJ, et al. Reorganisation of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, description of six new combinations and designations of Ehrlichi equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophilum. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 4.Ogden NH, Lindsay LR, Hanincová K, et al. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl Environ Microbiol. 2008;74:1780–1790. doi: 10.1128/AEM.01982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franzén P, Berg AL, Aspan A, Gunnarsson A, Pringle J. Death of a horse infected experimentally with Anaplasma phagocytophilum. Vet Rec. 2007;160:122–125. doi: 10.1136/vr.160.4.122. [DOI] [PubMed] [Google Scholar]
- 6.Madigan JE. Equine ehrlichiosis. Vet Clin North Am Equine Practice. 1993;9:423–428. doi: 10.1016/s0749-0739(17)30408-x. [DOI] [PubMed] [Google Scholar]
- 7.Lewis SR, Zimmerman K, Dascanio JJ, Pleasant RS, Witonsky SG. Equine granulocytic anaplasmosis: A case report and review. J Equine Vet Sci. 2009;29:160–166. [Google Scholar]
- 8.Madigan JE, Gribble D. Equine ehrlichiosis in nothern California: 49 cases (1968–1981) J Am Vet Med Assoc. 1987;190:445–448. [PubMed] [Google Scholar]
- 9.Lilliehook I, Egenvall A, Tvedten HW. Hematopathology in dogs experimentally infected with a Swedish granulocytic Ehrlichia species. Vet Clin Pathol. 1998;27:116–122. doi: 10.1111/j.1939-165x.1998.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 10.Lester SJ, Breitschwerdt EB, Collis CD, Hegarty BC. Anaplasma phagocytophilum infection (granulocytic anaplasmosis) in a dog from Vancouver Island. Can Vet J. 2005;46:825–827. [PMC free article] [PubMed] [Google Scholar]
- 11.Artursson K, Gunnarsson A, Wikström UB, Engvall EO. A serological and clinical follow-up in horses with confirmed equine granulocytic ehrlichiosis. Equine Vet J. 1999;6:473–477. doi: 10.1111/j.2042-3306.1999.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 12.Franzén P, Aspan A, Egenvall A, Gunnarsson A, Åberg L, Pringle J. Acute clinical, hematologic, serologic and polymerase chain reaction findings in horses experimentally infected with European strain of Anaplasma phagocytophilum. J Vet Intern Med. 2005;19:232–239. doi: 10.1892/0891-6640(2005)19<232:achsap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Berrington A, Moats R, Lester S. A case of Ehrlichia equi in an adult horse in British Columbia. Can Vet J. 1996;37:174–175. [PMC free article] [PubMed] [Google Scholar]
- 14.Cockwill KR, Taylor SM, Snead ECR, et al. Granulocytic anaplasmosis in three dogs from Saskatoon, Saskatchewan. Can Vet J. 2009;50:835–840. [PMC free article] [PubMed] [Google Scholar]