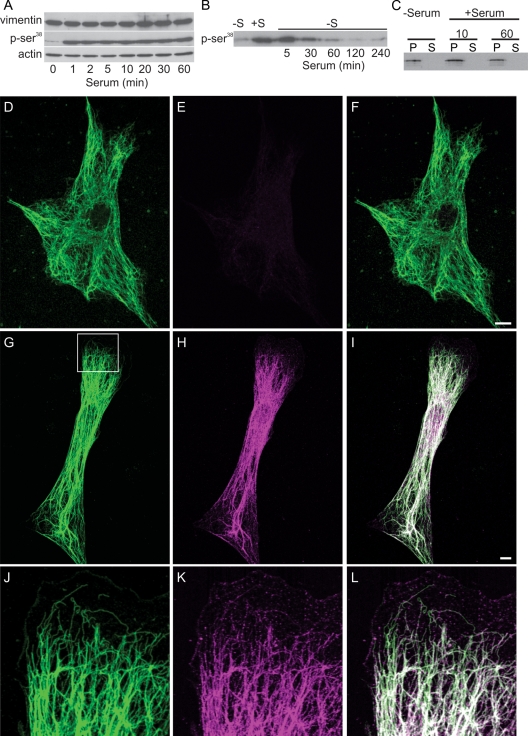
FIGURE 3:
Vimentin-Ser phosphorylation increases after serum addition. (A) Immunoblotting demonstrates that phosphorylation of vimentin Ser-38 increases dramatically within 1 min after serum addition and persists up to 60 min. Vimentin and actin are used as loading controls, and the pSer-38 blot has been exposed long enough to show the signal at 0 min. (B) Time course of vimentin–Ser-38 phosphorylation after serum activation followed by serum depletion. Cells were serum starved for 72 h (lane marked −S); serum was added for 30 min (+S), and then cells were replaced into serum-free medium and cell lysates prepared at the indicated times (5–240 min). (C) No differences were detected in the distribution of vimentin between the pellet and supernatant fractions after serum starvation (72 h) or 10 min and 60 min after serum stimulation. (D–F) Double-label immunofluorescence with anti-vimentin (D) and anti–vimentin pSer-38 (E) showing that this residue is minimally phosphorylated in a serum-starved 3T3 cell (72 h; F, overlay). (G–I) Staining with the anti–vimentin pSer-38 in a 3T3 cell 10 min after serum addition. Note that the entire VIF network stains with this phosphospecific antibody (G, anti-vimentin; H, anti–vimentin pSer-38; I, overlay; 10 min). (J–L) Higher magnification of the area denoted by the box in (G) demonstrates that pSer-38 is also found in particles and squiggles in the region where a lamellipodium is formed. Bars = 10 μm.