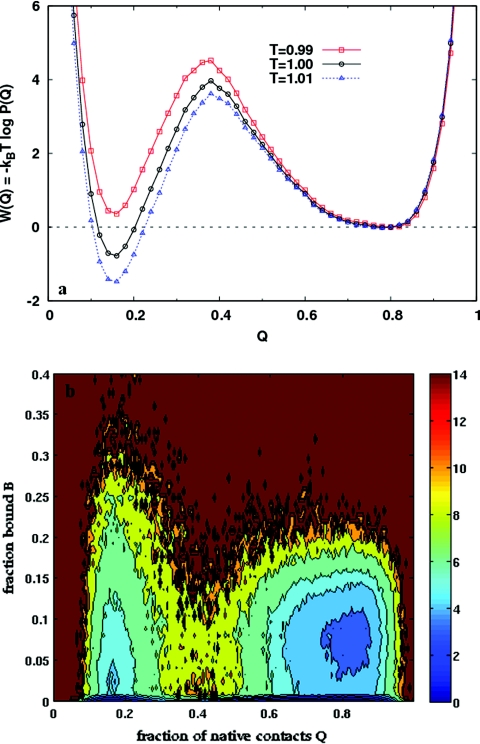
Figure 5.
(a) The PMF along Q in the case of weak coupling to the nanotube (λ = 0.5). Compared to the isolated protein at the same temperature [Fig. 3b], the free energy barrier is lowered and shifted to lower values of Q. Consequently, the folded basin becomes wider. (b) Free energy contours as a function of Q and B, the fraction of residues bound to the nanotube surface, for the λ = 0.5 case, at T = 0.99. This is the transition temperature for this system, corresponding to the peak of the specific heat vs temperature curve. The free energy is a minimum when the protein is completely unbound (B = 0), either folded (Q ∼ 0.8) or unfolded (Q ∼ 0.2). At low binding fractions (B ≲ 0.1), the protein can interconvert between the folded and unfolded states in a two state manner. At higher values of B the protein is unfolded.