Abstract
A hallmark event in neurodegenerative diseases is the accumulation in the brain of misfolded aggregated proteins, leading to neuronal dysfunction and disease. Compelling evidences suggest that misfolded proteins damage cells by inducing endoplasmic reticulum stress and alterations in calcium homeostasis. Changes in cytoplasmic calcium concentration lead to unbalances on several signaling pathways. Recent data suggest that calcium-mediated hyperactivation of calcineurin, a key phosphatase in the brain, trigers synaptic dysfunction and neuronal death, the two central events responsible for brain degeneration in neurodegenerative diseases. Therefore, blocking calcineurin activation might be a promising therapeutic strategy to prevent brain damage in neurodegenerative diseases.
Introduction
Neurodegenerative diseases are some of the most devastating disorders, affecting distinctive qualities of human beings, including abstract thinking, skilled movements, emotional feelings, cognition, memory, etc. This diverse group of diseases includes Alzheimer’s Disease (AD), Parkinson’s Disease (PD), Huntington’s Disease (HD) (and related poly-glutamine disorders including several forms of Spinocerebellar Ataxia), Transmissible Spongiform Encephalopathies (TSEs) and Amyotrophic Lateral Sclerosis (ALS) [1]. Compelling evidence suggests that cerebral accumulation of misfolded and aggregated proteins is a common and typical feature of these diseases and the most likely initiator of the pathogenesis [1,2]. Accumulation of misfolded proteins might lead to synaptic abnormalities and neuronal death, which ultimately produce brain dysfunction and disease [1]. Currently, there is no efficient therapy or pre-symptomatic diagnosis for any of these diseases. To identify novel strategies for intervention, it is essential to understand the mechanism of protein misfolding and the pathways by which misfolded aggregates induce neuronal death and synaptic alterations.
Endoplasmic Reticulum Stress and Calcium Alterations : A Common Pathway in Neurodegenerative Diseases
Recent evidences suggest that an early event following protein misfolding is a sustained endoplasmic reticulum (ER) stress, leading to alterations in the protein folding and clearance machinery, perturbations in calcium homeostasis, and activation of various intra-cellular signaling pathways [3–6].
Disruption of calcium homeostasis in the cell is probably the most adverse and immediate effect caused by ER stress produced by chronic accumulation of misfolded proteins [4]. Alterations in calcium homeostasis have been reported in different neurodegenerative disorders associated with accumulation of misfolded aggregates, including AD, PD, HD, ALS and TSE [3,4]. Compared to other types of cells, the effect becomes even more deleterious to neurons, because of the significant role of calcium waves in neuronal activity. Ca2+ plays an important role as a second messenger in different cellular signaling pathways, where the final outcome is control led by cellular calcium concentration [7]. For this reason, maintaining a specific Ca2+concentration in the cytoplasm is critical for normal neuronal biology. Cell utilizes different Ca2+ channels and ATP driven Ca2+ pumps to maintain a Ca2+ gradient and to stabilize the calcium homeostasis inside the cytoplasm [7]. ER functions as an intra-cellular Ca2+ storage. Ca2+ uptake into the ER from cytoplasm is guided by sarcoplasmic/ER Ca2+ ATPase (SERCA) and released via inositol 1,4,5-t riphosphate receptor (IP3R) or Raynodine receptor (RyR) [8]. Several studies have suggested increase of cytoplasmic Ca2+ due to ER stress in presence of misfolded proteins in various neurodegenerative diseases [3,4]. Previous work from our lab and others have reported the release of calcium from the ER to the cytoplasm when cells are exposed to misfolded prion protein [9]. Indeed, Ca2+ release appears to be one of the first adverse effect s after prion infect ion in cells. Recent evidences strongly suggest that at least a major source of elevated Ca2+ in the cytoplasm of prion infected cells is leakage from the ER [10].
Among the consequences of protein misfolding mediated through ER stress and alterations in calcium homeostasis are changes on the activity of various kinases and phosphatases that play a critical role in maintaining cellular functioning. In this review we will focus on the role of one particular brain phosphatase, called calcineurin (CaN), activated by ER stress during formation of misfolded aggregates.
Calcineurin Biology
CaN is a Ca2+/Calmodulin dependent serine/threonine phosphatase highly abundant in mammalian brain tissue [11]. Insensitivity of CaN towards heat stable inhibitor proteins and its ability to preferentially dephosphorylate the α-subunit of phosphorylase kinase distinguish CaN from phosphatase type 1 and classify it under phosphatase type 2 (PP2). Ca2+ dependency of CaN sub-classify this enzyme under phosphatase type 2B (PP2B) and distinguish it from spontaneously active PP2A or Mg2+ -dependent PP2C [12–14]. Since the identification of CaN in late 1970s and ground-breaking discovery that it is the target of immunosuppressive drugs cyclosporine A and FK506 [15–17], extensive studies have been done to determine the structure and function of CaN. There are a large number of comprehensive review articles available describing the function of this protein [18,19]. Therefore, in the current article we will not go into details of the structure and function of CaN, instead we will focus on its potential role in neurodegeneration induced by misfolded proteins.
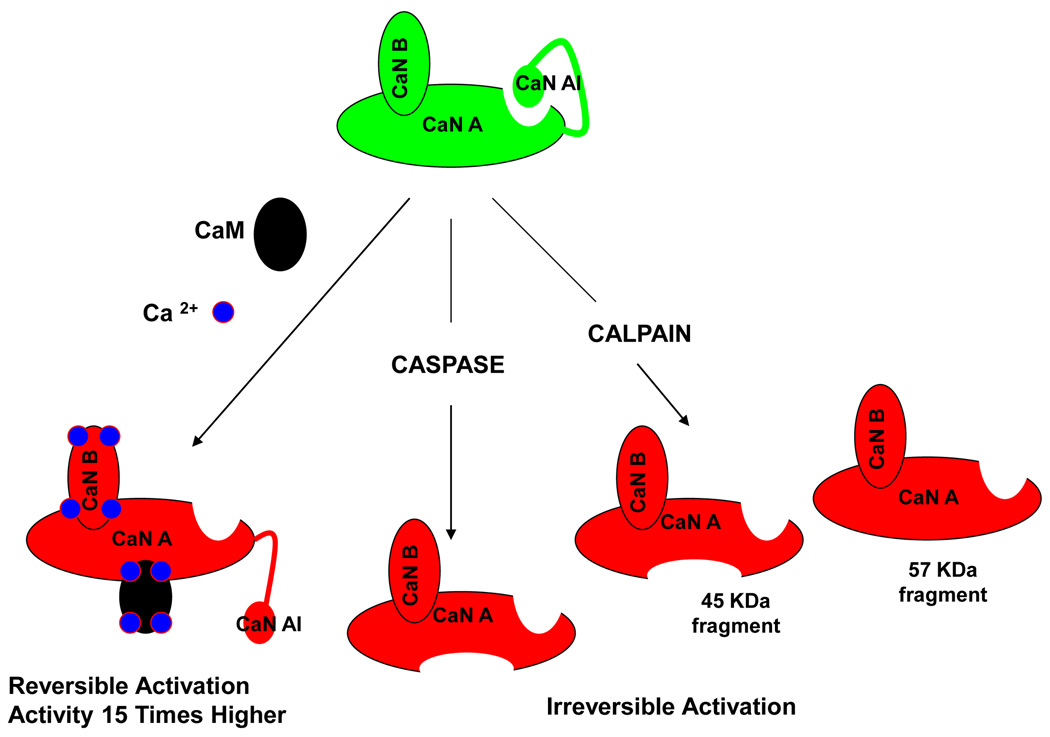
CaN is a heterodimer composed of a 60 KDa catalytic subunit (calcineurin A; CnA) and an 18 KDa regulatory subunit (calcineurin B; CnB) [18]. Al though the amino acid sequence of the catalytic domain is homologous to other serine/threonine protein phosphatases, the presence of three other regulatory domains in the carboxy-terminal of the subunit A distinguish CaN from others [14]. These domains are the CnB binding domain, the calmodulin-binding domain and the auto-inhibitory domain (CnAI) that binds to the active site in the absence of Ca2+/Calmodulin, inhibiting the enzyme activity. Ca2+/Calmodul in complex binds to subunit A with very high affinity followed by release of the auto-inhibitory domain from the active site, leading to enzymatic activation [20,21]. Kinetic studies revealed that there is roughly 15-times increase in the enzyme activity in presence of Ca2+/Calmodulin complex (Fig. 1) [22]. CaN can be phosphorylated in the calmodulin-binding region. However, calmodul in can still bind and activate CaN in the phosphorylated state [23].
Figure 1. Reversible and Irreversible activation of calcineurin.
CaN can be activated reversibly in presence of Ca2+/CaM up to 15 times. This seems to happen during chronic elevation of Ca2+ in the cytoplasm resulting from ER stress after exposure to misfoled proteins. CaN can also be activated irreversibly by proteolytic cleavage of the CnAI and CaM binding domains. This cleaved form of the enzyme is no longer sensitive to Ca2+/CaM and thus constitutively active.
The mature CnB subunit is missing the initiator methionine, and the α-amino group of glycine at position 2 is modified by acylated myristric acid [18]. This post-translational modification is conserved throughout evolution suggesting a crucial physiological role. However non-myristoylated CaN displays similar biological function [18].
Besides activation by calcium, CaN might be activated at least in two other ways involving proteolysis (Fig. 1). The first one implicates caspases. Caspase-mediated cleavage of the CaN auto-inhibitory domain and the calmodulin-binding domain renders the enzyme constitutively active and insensitive to Ca2+/Calmodulin [24]. However, due to the fact that activation of caspases is a downstream process during apoptosis, the biological significance of this cleavage is questionable. Another, seemingly more relevant, way of constitutive CaN activation is partial proteolysis by Ca2+-dependent cysteine protease called calpain (Fig. 1) [25]. In vi tro MALDI-TOF analysis has identified three different carboxy-terminal truncated forms of CaN after calpain cleavage corresponding to 45, 48 and 57 kDa fragments [25]. Among them the 45 kDa fragment does not contain either the calmodulin-binding domain or the auto-inhibitory domain leading to a Ca2+/Calmodulin insensitive, constitutively active enzyme [26]. Samples from human Alzheimer’s brain showed the presence of the 57 kDa fragment, missing only the auto-inhibitory domain [27]. This fragment is still Ca2+/Calmodulin sensitive. However, the truncation remarkably enhances the enzyme activity [27].
Calcineurin role in Neuronal Homeostasis
CaN is abundant in the cytosol, pre- and post-synaptic terminals in neurons [28]. The fact that CaN is the only Ca2+ -dependent phosphate present in neurons suggest that it might play a crucial role in maintenance of cellular homeostasis under Ca2+ oscillations. In fact, the putative role of CaN in neuronal activity has been studied extensively [19,28].
Ca2+ influx in the neuronal cytosol activates a bunch of proteins to initiate the downstream signaling mechanism. Due to very high affinity (0.1–1nM) for Ca2+/CaM and co-localization with N-methyl-D-aspartate receptor, CaN activates promptly after cytoplasmic Ca2+ influx [21,29]. Upon activation, CaN contributes to inhibit further Ca2+ influx into the cytosol. CaN performs this task in multiple ways. It slows down the Ca2+ influx from plasma membrane by weakening voltage-gated Ca2+ channels as well as regulates Ca2+-induced Ca2+ release from the ER by negatively controlling IP3 and RyR through dephosphorylation [30–33].
The importance of CaN is not restricted to the regulation of Ca2+ influx into the cytosol, but it has also been shown that CaN plays an important role in modulating gene expression. In neurons one of the major transcription factor s working under the control of CaN is cAMP–response element binding protein (CREB) [18,29,34]. CREB is phosphorylated by Ca2+-dependent and independent protein kinases and translocate into the nucleus followed by CREB dependent gene expression [34]. CaN works like a switch that shuts off this gene expression process by dephosphorylating CREB [19,28,34]. Another important transcription factor regulated by CaN is Nuclear Factor of Activated T-Cell (NFAT). Dephosphorylated by CaN, NFAT4 translocate into the nucleus and induces the expression of neutrophilin and netrin dependent gene expression required for axonal outgrowth [35,36]. Transcript ion factor Myocyte Enhancer Factor 2 (MEF2) has also been shown to be modulated by CaN [37,38]. Dephosphorylated by CaN, MEF2 switches from a sumoylated to an acetylated form, which activates the transcription factor. As a result, several genes, including arc and synGAP, are activated followed by inhibition of dendritic claw development during neural morphogenesis [37,38]. However, CaN mediated dephosphorylation of MEF2 is required for it sprosurvival signaling. Infact, phosphorylation of MEF2 at the CaN target site (Ser408) is implicated in neurotoxin induced cell death [39].
Calcineurin and Neurodegeneration
Over-activated CaN is implicated in a reversible (operated by post-translational modifications) neuronal apoptotic pathway involving Bcl-2 family proteins [7,40]. Hyper-activation of CaN due to chronic increase of cytoplasmic Ca2+, reduces the phosphorylation of pro-apoptic BAD [41–43], which in the normal phosphorylated state is associated with scaffolding protein 14-3-3 (Fig. 2). However, dephosphorylated BAD disassociate from 14-3-3 and interact with Bcl-x and other Bcl2 family proteins located in the mitochondrial membrane [41,42,44]. Interaction of Bcl-x with BAD weakens its normal anti-apoptotic activity rendering the cells predisposed to apoptosis (Fig. 2) [44]. Various studies have shown low level phosphorylated BAD in cells treated with different misfolded proteins and in the brains of mouse models of several neurodegenerative disorders [45,46]. We have shown that basal level of BAD phosphorylation can be recovered by pharmacological normalization of CaN activity in a mouse model of prion disease, using the well-established CaN inhibitor tacrolimus or FK506 [47]. In fact, our data strongly suggest that normalization of CaN activity can significantly prevent neuronal loss, leading to increased survival in prion infected mice [47].
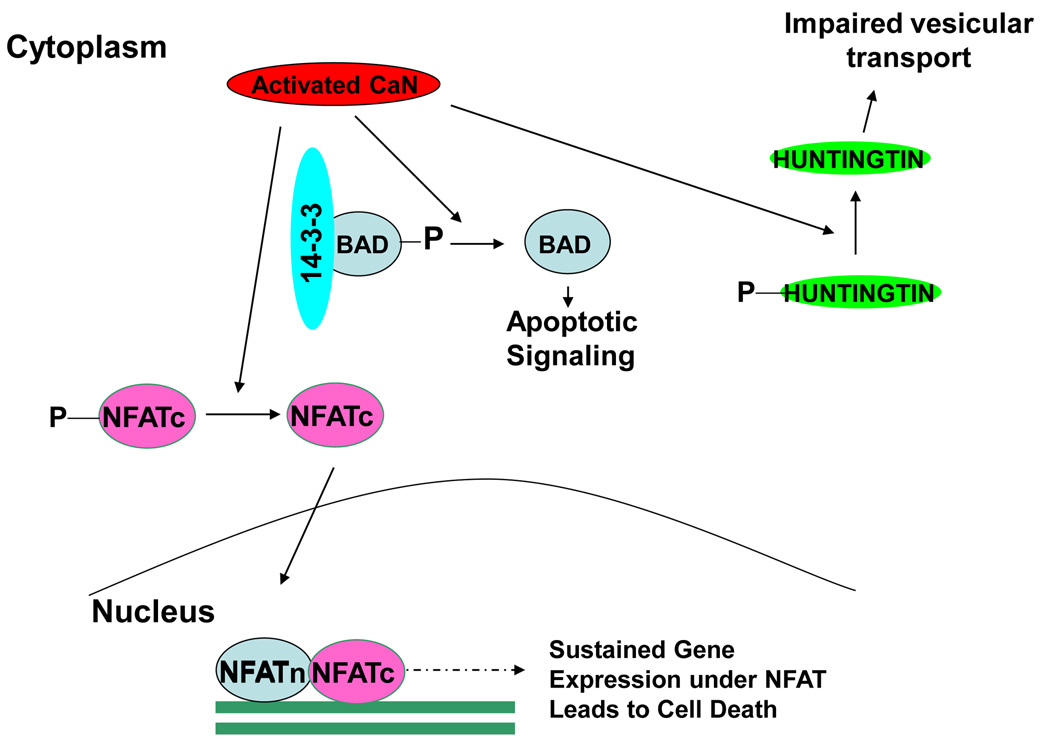
Figure 2. Role of chronically activated CaN in neuronal death.
Chronically activated CaN has been implicated in neuronal death at least in three major ways: (1) Dephosphorylation of BAD, releasing it from the scaffolding protein 14-3-3, leading to apoptotic activity; (2) Aberrant dephosphorylation of the cytoplasmic subunit of NFAT (NFATc) induces its translocation to the nucleus where interact with NFATn, leading to chronic induction of NFAT regulated genes; (3) Dephosphorylation of Hunt ingtin result in a partial loss of function of this protein leading to impaired vesicular transport, especially of various growth factors, including BDNF.
A recent report suggested that CaN might also be involved in neurodegeneration through NFAT (nuclear factor of activated T-cell) signaling (Fig. 2). This pathway was proposed to operate in Aβ mediated cell death in AD [48]. NFAT complex is a transcription factor consisting of at least two different components [49]. The inducible component, called NFATn, is always in the nucleus. The other component, called NFATc, is present in the cytoplasm in its native phosphorylated state. Dephosphorylated by activated CaN, NFATc translocate into the nucleus, complexes with NFATn and induces target gene expression (Fig. 2). Chronically activated CaN in presence of Aβ induces an aberrant activation of NFATc4 (the neuronal isoform of NFAT) [48]. Consistent presence of activated NFAT in the nucleus is sufficient to induce morphological neurodegenerative abnormalities, including dystrophic neurites, dendrite simplification and dendritic spine loss [48].
Recently, CaN was suggested as an important therapeutic target for Huntington’s Disease (HD) (Fig. 2) [50]. Huntingtin protein, in its normal phosphorylated state positively regulates vesicular transport, particularly of neurotrophins such as Brain Derived Neuronal Growth Factor (BDNF). This function is compromised in HD patients causing a decrease in the neurotrophic support and subsequently followed by neuronal death [51]. Hyperactivated CaN has been implicated in aberrant dephosphorylation of Huntingtin resulting in deregulation of BDNF transport causing neurodegeneration [50,52]. In fact, genetic and pharmacological inhibition of CaN activity has been shown to restore neuronal death in cells derived from a HD mouse model [50]. Further studies showed that over expression of a negative regulator of CaN can also protects mutant huntingtin toxicity [53].
Calcineurin and Synaptic Dysfunction
Although, neuronal death is the final step responsible for the fatal outcome of neurodegenerative disorders, the pathological cascade and clinical symptoms begin with synaptic de-regulation well before neuronal death is observed [54–56]. In regular physiological conditions CaN plays a very important role in maintaining synaptic function under the Ca2+ spikes in neurons [19]. CaN is abundant in both the pre-synaptic and post-synaptic terminals. In the pre-synaptic terminals it plays a major role in neuro-transmitter release by regulating endo- and exo-cytosis (Fig. 3) [28]. On the other hand, in the post-synaptic terminals CaN regulates ion channels [30,57]. Therefore, it is not difficult to imagine that aberrant CaN activity will de-regulate the synaptic mechanism as a whole. Indeed, several studies using CaN transgenic and knock out animal models have shown that CaN plays a key role in long term potentiation (LTP) and memory formation [58–60].
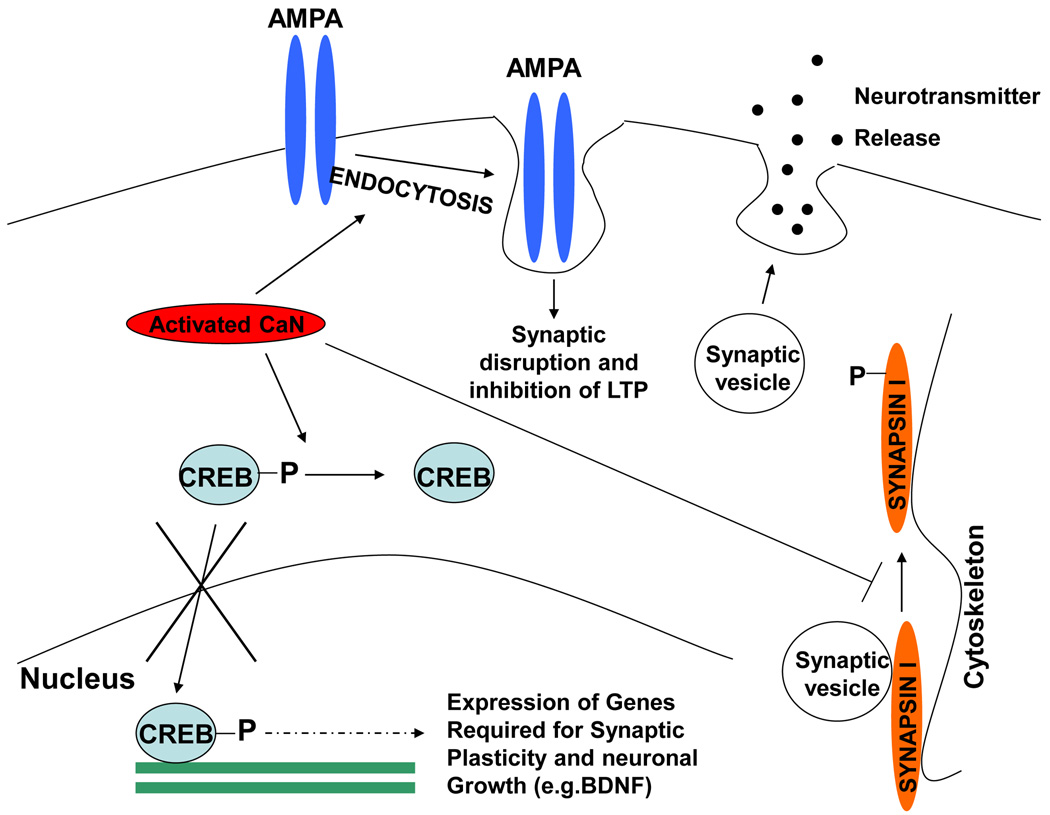
Figure 3. Role of chronically activated CaN in synaptic dysfunction.
CaN has been implicated in synaptic abnormalities during neurodegenerative disorders at least in three different ways: (1) Activated CaN dephosphorylates CREB inhibiting its translocation to the nucleus, resulting in the abnormal shut off of CREB regulated gene expression required for neuronal growth and synaptic plasticity; (2) CaN mediates the internalization of AMPA receptor bound to misfolded proteins (particularly Aβ oligomers) inducing synaptic disruption and inhibition of long term potentiation; (3) Inhibition of neurotransmitter release by abrogating synaptic vesicle transport through dephosphorylaton of synapsin I.
It has been suggested that CaN-mediated endocytocis of AMPA (α-amino-3hydroxy-5methyl-4-isoxazolepropionic acid) receptor is involved in Aβ induced synaptic disruption [61], which subsequently inhibit LTP and induce memory loss. In this model, Aβ oligomers bind to dendritic spines that express surface AMPA receptors. CaN mediates an endocytotic process that is responsible for the rapid internalization of Aβ oligomers bound to surface AMPA receptor subunits [61], which then colocalize with cpg2, a molecule located specifically at the postsynaptic endocytic zone of excitatory synapses that plays an important role in activity-dependent glutamate receptor endocytosis. Importantly, CaN inhibitors prevent oligomer-induced surface AMPA receptors and spine loss. An independent study also showed that soluble Aβ mediated dendritic spine loss correlated with chronic activation of CaN [62]. Strikingly, acute inhibition of CaN reversed the intermediate and long term recognition memory deficits in an AD mouse model [63].
CaN not only regulates synaptic function by post-translation modification of target proteins, it also plays a major role in controlling gene expression required for synaptic plasticity. As described before, an important CaN target is the transcription factor CREB (Fig. 3). CREB-induced gene expression is required for different proteins involved in neuronal growth and survival, including BDNF and its receptor tropomyosin related kinase B (trkB) [64–68]. Phosphorylated CREB translocates into the nucleus and exert its transcription factor activity (Fig. 3). However, chronically activated CaN has been shown to dephosphorylate and inactivate CREB, subsequently shutting down the CREB-dependent gene expression [34], leading to loss of synaptic plasticity. This effect has been reported to occur in the presence of misfolded Aβ and prions [45–47,69]. Even in wild type mouse, CaN inhibition has been shown to increase dendritic branching and spine density [70]. Our own data suggest that rescue of optimum CREB phosphorylation, by CaN inhibition is able to slow down behavioral impairment of prion infected animals even at the clinical stage of the disease [47]. However it is important to remember that basal level of CaN activity is important for CREB dependent gene expression [71,72]. Focal bead-like swelling in dendrites and axons is one of the major features of synaptic pathology in neurodegenerative disorders. Interestingly, CaN inhibition has been shown to reverse these neuritic beading and rescue structural disruption of neuronal network in presence of Aβ [73]. In addition, as described earlier CaN regulates different proteins involved in neurotransmitter release, including synapsin, synaptotagmin, rabphilin2A, synaptobrevin and dephosphins [74]. Among them synapsin I is of major importance because of its role in vesicle trafficking (Fig. 3). In resting conditions, synapsin I attaches the vesicle to the actin based cytoskeleton. During excitation, phosphorylated by different kinases, it detaches from the vesicles facilitating neurotransmitter release [75]. CaN and PP2A reverse this process by dephosphorylation, contributing to maintain a proper balance [76].
To summarize, CaN is involved in the maintenance of synaptic structure and function in various ways. Therefore any abnormality in the CaN function is expected to be amplified as an overall deregulation of synaptic activity. Thus, restricting CaN activity to an optimum level can be a potential therapeutic approach to prevent synaptic dysfunction, which appears to be an early event in different neurodegenerative disorders.
Concluding Remarks
Understanding the pathways by which misfolded proteins cause neurodegeneration and disease is essential to develop much needed efficient treatments for neurodegenerative disorders. The available evidence indicates that accumulation of misfolded proteins causes ER stress and alterations in calcium homeostasis [3–6]. In response to the damage, cells engage the unfolded protein response to attempt correcting the negative consequences of ER stress [77,78]. Sustained stress leads to neurodegeneration in the form of synaptic dysfunction and neuronal apoptosis. A particularly negative outcome of ER stress is the release of calcium to the cytoplasm, leading to a drastic disbalance of essential signaling pathways. Among the many proteins affected by alterations in calcium homeostasis is CaN, a key phosphatase in the brain. Recent exciting data in various neurodegenerative disease have implicated changes on CaN activity in the cellular pathways leading to synaptic loss and neuronal death. More importantly, administration of CaN inhibitors to various mice models of neurodegenerative disease appear to have therapeutic benefit [47,50,52,61,63,69]. Additional research in this area may contribute to increase our understanding of the molecular basis of neurodegeneration and the development of much needed therapeutic strategies for neurodegenerative diseases.
Acknowledgement
This work was supported in part by NIH grant R01NS050349.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest have been highlighted as • of special interest •• of outstanding interest.
- 1.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 2.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 3.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cel l Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 4.Hetz CA, Soto C. Stressing out the ER: a role of the unfolded protein response in prion-related disorders. Curr Mol Med. 2006;6:37–43. doi: 10.2174/156652406775574578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 6.Lehotsky J, Kaplan P, Babusikova E, Strapkova A, Murin R. Molecular pathways of endoplasmic reticulum dysfunctions: possible cause of cell death in the nervous system. Physiol Res. 2003;52:269–274. [PubMed] [Google Scholar]
- 7.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- 9.Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres M, Castillo K, Armisen R, Stutzin A, Soto C, Hetz C. Prion protein misfolding affects calcium homeostasis and sensitizes cells to endoplasmic reticulum stress. PLoS One. doi: 10.1371/journal.pone.0015658. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Xiong F, Kong S, Ogawa T, Kobayashi M, Liu JO. Distinct tissue and cellular distribution of two major isoforms of calcineurin. Mol Immunol. 1997;34:663–669. doi: 10.1016/s0161-5890(97)00054-0. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P, Cohen PT. Protein phosphatases come of age. J Biol Chem. 1989;264:21435–21438. [PubMed] [Google Scholar]
- 13.Ingebritsen TS, Stewart AA, Cohen P. The protein phosphatases involved in cellular regulation. 6. Measurement of type-1 and type-2 protein phosphatases in extracts of mammalian tissues; an assessment of their physiological roles. Eur J Biochem. 1983;132:297–307. doi: 10.1111/j.1432-1033.1983.tb07362.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 16.Lepre CA, Thomson JA, Moore JM. Solution structure of FK506 bound to FKBP-12. FEBS Lett. 1992;302:89–96. doi: 10.1016/0014-5793(92)80292-o. [DOI] [PubMed] [Google Scholar]
- 17.White DJ, Calne RY, Plumb A. Mode of action of cyclosporin A: a new immunosuppressive agent. Transplant Proc. 1979;11:855–859. [PubMed] [Google Scholar]
- 18.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 19.Shibasaki F, Hallin U, Uchino H. Calcineurin as a multifunctional regulator. J Biochem. 2002;131:1–15. doi: 10.1093/oxfordjournals.jbchem.a003063. [DOI] [PubMed] [Google Scholar]
- 20.Kissinger CR, Parge HE, Knighton DR, Lewis CT, Pelletier LA, Tempczyk A, Kalish VJ, Tucker KD, Showalter RE, Moomaw EW, et al. Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature. 1995;378:641–644. doi: 10.1038/378641a0. [DOI] [PubMed] [Google Scholar]
- 21.Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li HC. Activation of brain calcineurin phosphatase towards nonprotein phosphoesters by Ca2+, calmodulin, and Mg2+ J Biol Chem. 1984;259:8801–8807. [PubMed] [Google Scholar]
- 23.Hashimoto Y, King MM, Soderling TR. Regulatory interactions of calmodulin-binding proteins: phosphorylation of calcineurin by autophosphorylated Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1988;85:7001–7005. doi: 10.1073/pnas.85.18.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukerjee N, McGinnis KM, Park YH, Gnegy ME, Wang KK. Caspase-mediated proteolytic activation of calcineurin in thapsigargin-mediated apoptosis in SH-SY5Y neuroblastoma cells. Arch Biochem Biophys. 2000;379:337–343. doi: 10.1006/abbi.2000.1889. [DOI] [PubMed] [Google Scholar]
- 25.Wu HY, Tomizawa K, Matsui H. Calpain-calcineurin signaling in the pathogenesis of calcium-dependent disorder. Acta Med Okayama. 2007;61:123–137. doi: 10.18926/AMO/32905. [DOI] [PubMed] [Google Scholar]
- 26.Manalan AS, Klee CB. Activation of calcineurin by limited proteolysis. Proc Natl Acad Sci U S A. 1983;80:4291–4295. doi: 10.1073/pnas.80.14.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong CX. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem. 2005;280:37755–37762. doi: 10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- 28.Mansuy IM. Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun. 2003;311:1195–1208. doi: 10.1016/j.bbrc.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard MJ, Klee CB. Calmodulin binding by calcineurin. Ligand-induced renaturation of protein immobilized on nitrocellulose. J Biol Chem. 1987;262:15062–15070. [PubMed] [Google Scholar]
- 30.Armstrong DL. Calcium channel regulation by calcineurin, a Ca2+-activated phosphatase in mammalian brain. Trends Neurosci. 1989;12:117–122. doi: 10.1016/0166-2236(89)90168-9. [DOI] [PubMed] [Google Scholar]
- 31.Burley JR, Sihra TS. A modulatory role for protein phosphatase 2B (calcineurin) in the regulation of Ca2+ entry. Eur J Neurosci. 2000;12:2881–2891. doi: 10.1046/j.1460-9568.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Yakel JL. Calcineurin modulates G protein-mediated inhibition of N-type calcium channels in rat sympathetic neurons. J Neurophysiol. 1997;78:1161–1165. doi: 10.1152/jn.1997.78.2.1161. [DOI] [PubMed] [Google Scholar]
- 33.Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ. Stimulation of 5-HT(2) receptors in prefrontal pyramidal neurons inhibits Ca(v)1.2 L type Ca(2+) currents via a PLCbeta/IP3/calcineurin signaling cascade. J Neurophysiol. 2002;87:2490–2504. doi: 10.1152/jn.00843.2001. [DOI] [PubMed] [Google Scholar]
- 34.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 35.Graef IA, Wang F, Charron F, Chen L, Neilson J, Tessier-Lavigne M, Crabtree GR. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–670. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 36.Groth RD, Mermelstein PG. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcript ion: a role for the transcript ion factor NFATc4 in neurotrophin-mediated gene expression. J Neurosci. 2003;23:8125–8134. doi: 10.1523/JNEUROSCI.23-22-08125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- 38.Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 39.Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, Blair LA, Marshall J, Mao Z. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/s0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 40.Klumpp S, Krieglstein J. Serine/threonine protein phosphatases in apoptosis. Curr Opin Pharmacol. 2002;2:458–462. doi: 10.1016/s1471-4892(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 41.Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 42.Shou Y, Li L, Prabhakaran K, Borowitz JL, Isom GE. Calcineurin-mediated Bad translocation regulates cyanide-induced neuronal apoptosis. Biochem J. 2004;379:805–813. doi: 10.1042/BJ20031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Omori K, Suzukawa J, Inagaki C. Calcineurin-mediated BAD Ser155 dephosphorylation in ammonia-induced apoptosis of cultured rat hippocampal neurons. Neurosci Lett. 2004;357:73–75. doi: 10.1016/j.neulet.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 44.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 45.Agostinho P, Lopes JP, Velez Z, Oliveira CR. Overactivation of calcineurin induced by amyloid-beta and prion proteins. Neurochem Int. 2008;52:1226–1233. doi: 10.1016/j.neuint.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7:824–835. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mukherjee A, Morales-Scheihing D, Gonzalez-Romero D, Green K, Taglialatela G, Soto C. Calcineurin inhibition at the clinical phase of prion disease reduces neurodegeneration, improves behavioral alterations and increases animal survival. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001138. e1001138. This study shows that calcineurin activation induced by prion protein misfolding triggers neuronal death and that treatment of prion infected mice at the clinical phase of the disease with a calcineurin inhibitor slow down deterioration, reduces neurodegeneration and increases animal survival.
- 48. Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. This study show that soluble Abeta oligomers lead to activation of calcineurin, which in turn activates the transcriptional factor nuclear factor of activated T cells (NFAT). Activation of these signaling pathways, leads to dystrophic neurites, dendritic simplification, and dendritic spine loss in both neurons in culture and in the adult mouse brain.
- 49.Nguyen T, Di Giovanni S. NFAT signaling in neural development and axon growth. Int J Dev Neurosci. 2008;26:141–145. doi: 10.1016/j.ijdevneu.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pineda JR, Pardo R, Zala D, Yu H, Humbert S, Saudou F. Genetic and pharmacological inhibition of calcineurin corrects the BDNF transport defect in Huntington's disease. Mol Brain. 2009;2:33. doi: 10.1186/1756-6606-2-33. This study shows the involvement of calcineurin in huntingtin biological function and its possible role in huntington’s disease.
- 51.Humbert S, Saudou F. Huntington's disease: intracellular signaling pathways and neuronal death. J Soc Biol. 2005;199:247–251. doi: 10.1051/jbio:2005026. [DOI] [PubMed] [Google Scholar]
- 52.Pardo R, Colin E, Regulier E, Aebischer P, Deglon N, Humbert S, Saudou F. Inhibition of calcineurin by FK506 protects against polyglutamine-huntingtin toxicity through an increase of huntingtin phosphorylation at S421. J Neurosci. 2006;26:1635–1645. doi: 10.1523/JNEUROSCI.3706-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ermak G, Hench KJ, Chang KT, Sachdev S, Davies KJ. Regulator of calcineurin (RCAN1-1L) is deficient in Huntington disease and protective against mutant huntingtin toxicity in vitro. J Biol Chem. 2009;284:11845–11853. doi: 10.1074/jbc.M900639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 55.Mallucci GR. Prion neurodegeneration: starts and stops at the synapse. Prion. 2009;3:195–201. doi: 10.4161/pri.3.4.9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wishart TM, Parson SH, Gillingwater TH. Synaptic vulnerability in neurodegenerative disease. J Neuropathol Exp Neurol. 2006;65:733–739. doi: 10.1097/01.jnen.0000228202.35163.c4. [DOI] [PubMed] [Google Scholar]
- 57.Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- 58. Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. This landmark study describes the key role of calcineurin in memory formation.
- 59.Mal leret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 60.Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 61. Zhao WQ, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M, Wolfe AL, Seager MA, et al. Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem. 2010;285:7619–7632. doi: 10.1074/jbc.M109.057182. This study elucidate a novel putative mechanism by which Abeta oligomers may cause synaptic disruption, involving binding and endocytosis of AMPA receptor, which in turn is controlled by calcineurin phosphatase activity.
- 62.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. This study shows that memory deficits in an Alzheimer’s disease mice model can be prevented and reversed by calcineurin inhibition.
- 64.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 65.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 66.Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 68.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 69.Dineley KT, Kayed R, Neugebauer V, Fu Y, Zhang W, Reese LC, Taglialatela G. Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J Neurosci Res. 2010;88:2923–2932. doi: 10.1002/jnr.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Spires-Jones TL, Kay K, Matsouka R, Rozkalne A, Betensky RA, Hyman BT. Calcineurin inhibition with systemic FK506 treatment increases dendritic branching and dendritic spine density in healthy adult mouse brain. Neurosci Lett. doi: 10.1016/j.neulet.2010.10.033. in press. This study shows acute treatment with FK506 of healthy adult mice increases both the branching pat terns and dendritic spine density of cortical neurons. These results indicate that calcineurin plays an important role in both the function and structure of the adult brain.
- 71.Kingsbury TJ, Bambrick LL, Roby CD, Krueger BK. Calcineurin activity is required for depolarization-induced, CREB-dependent gene transcription in cortical neurons. J Neurochem. 2007;103:761–770. doi: 10.1111/j.1471-4159.2007.04801.x. [DOI] [PubMed] [Google Scholar]
- 72.Lam BY, Zhang W, Enticknap N, Haggis E, Cader MZ, Chawla S. Inverse regulation of plasticity-related immediate early genes by calcineurin in hippocampal neurons. J Biol Chem. 2009;284:12562–12571. doi: 10.1074/jbc.M901121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- 75.Hosaka M, Hammer RE, Sudhof TC. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron. 1999;24:377–387. doi: 10.1016/s0896-6273(00)80851-x. [DOI] [PubMed] [Google Scholar]
- 76.Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC, Jr, Greengard P, Czernik AJ. Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci. 2001;21:7944–7953. doi: 10.1523/JNEUROSCI.21-20-07944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]