Abstract
Mesenchymal stem cells (MSCs) have elicited a great clinical interest, particularly in the areas of regenerative medicine and induction of tolerance in allogeneic transplantation. Previous reports demonstrated the feasibility of transplanting MSCs, which generates new prospects in cellular therapy. Recently, injection of MSCs induced remission of steroid-resistant acute graft-versus-host disease (GVHD). This review summarizes the knowledge and possible future clinical uses of MSCs.
Keywords: mesenchymal stem cells, plasticity, immunomodulation, cancer, gene therapy
Abbreviations
- BM
Bone Marrow
- GVHD
graft-versus-host disease
- HSC
hematopoietic stem cells
- PMNC
peripheral mononuclear cells
- MSCs
mesenchymal stem cells
- NOD/SCID
nondiabetic severe combined immune deficiency
- OI
osteogenesis imperfecta
Introduction
Isolation of bone marrow (BM) cells that could form new bone when transplanted to an ectopic site was demonstrated using the guinea pig model [1, 2]. These derived stromal cells, named mesenchymal stem cells (MSCs), were expanded from adherent stromal cells in bone marrow culture. The results were confirmed later in both rabbit and rat bone marrow cells [2–4].
MSCs are adult clonal multipotential stem cells localized in the medullary stroma [5–7]. The human body contains many stem cells, i.e. hematopoietic (HSC) [8], neural [9], epithelial [10, 11] and embryonic stem cells [12]. MSCs do not fulfill all true stem cell criteria. In contrast to HSC, single MSCs cannot regenerate a whole tissue compartment, and they do not have indefinite self renewal capacity.
MSC cells represent 1/10,000 to 1/100,000 of all mononuclear cells in the BM, and they can be expanded 500-fold through as many as 50 generations to produce billions of cells [13–16]. Colonies derived from a single MSC vary to some extent in differentiation capacity and expansion potential [17–20]. Entry of MSC into senescence is almost undetectable, and they lose their stem cell characteristics and differentiation potential from the sixth passage onwards [21].
Haynesworth et al developed a reliable in vivo bone-forming assay and were able to isolate and expand human MSCs for therapeutic purposes [22].
The ability to expand MSCs in vitro for clinical applications has recently facilitated the development of clinical trials designed to assess the safety, feasibility, and efficacy of transplanting MSCs for a variety of diseases [14]. Neither toxicity nor malignancy was associated with infusion of expanded autologus MSCs into patients with advanced breast cancer, with Hurler syndrome, or with metachromatic leukodystrophy [23–25].
In this review we will discuss the following:
Characterstics of MSCs
MSCs isolation and culture expansion
Transplantibility and engrafment of MSCs
Role of MSCs in support of hematopoiesis
MSCs plasticity, differentiation, possible uses in regenerative medicine and treatment of various diseases
Role of MSCs in immunomodulation
MSCs and solid organ graft
Role of MSCs in irradiation injuries or burns
MSCs in gene therapy
MSCs in cancer
Characteristics of MSCs
MSCs are unspecialized cells that lack tissue-specific characteristics and can maintain their undifferentiated phenotype. Under the influence of specific biological signals, MSCs can differentiate into specialized cells with a phenotype that is fully distinct from that of the precursor.
MSCs express neither the hematopoietic markers CD34, CD45, CD14, CD11 (7,26), nor the co-stimulatory molecules CD 80, CD 86, CD40, CD 40 ligand and CD 154 (27). MSCs are positive for CD73, CD105 and CD90 (28). MSCs express adhesion molecules, including VCAM (CD 106), ICAM (CD54), and LFA-3 (29). It has been demonstrated that human MSC MHC (HLA-DR) is localized in the submembranous space near the nucleolus (30), but cell surface expression of class I and class II MHC requires activation by interferon-γ (IFN-γ) (27, 31).
MSCs secrete respectively Interleukin-6 (IL-6), IL-7, IL-11, IL-12, IL-14, IL-15, leukemia inhibitory factor (LIF), macrophage colony-stimulating factor (M-CSF), stem cell factor (SCF), and flt-3 ligand [32].
Minimal criteria for defining multipotent mesenchymal stromal cells according to the International Society for Cellular Therapy are the ability to regenerate and differentiate into tissues of mesodermal origin (osteocytes, adipocytes and chondrocytes), and the absence of expression of haemopoietic molecules [28].
MSC isolation and culture expansion
MSCs have been isolated from adipose tissue, fetal liver, blood, lung, postnatal marrow, cord blood, brain, spleen, kidney, bone marrow, muscle, thymus, pancreas, and from human peripheral blood mobilized with Granulocyte-Colony stimulating factor (G-CSF) [33–36]. However, long-term cultures of MSCs can be generated only from blood vessels [37].
Human MSCs are isolated from the total nucleated cell population in a BM aspirate, which is often harvested from the superior iliac crest of the pelvis, after separation by discontinuous density gradient centrifugation [6, 38, 39]. Mononuclear cells (MNC) are then cultured in a medium, such as Dulbecco's modified Eagle's medium (DMEM), or alpha MEM (α-MEM) supplemented with 10% fetal calf serum (FCS), platelet-rich plasma (PRP), or a commercial substitute of human serum [15, 40, 41].
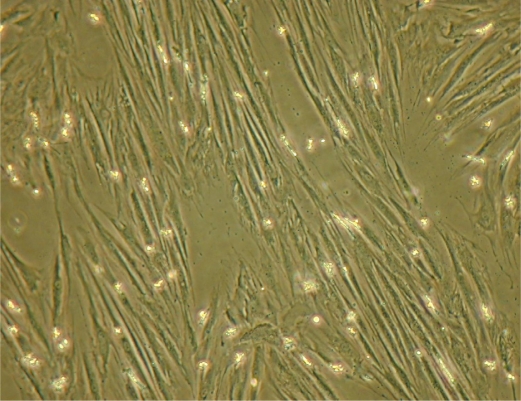
In culture, the non-adherent MNC are washed away to leave behind small, adherent fibroblast-like cells. Cultures have an initial lag phase of three to five days [42], followed by rapid proliferation with an average initial doubling time ranging from 12 to 24 h and varying from one donor to another [15]. MSCs have a spindle shape (Figure 1), and they can be expanded for about three weeks. At confluence, MSCs enter a stationary phase [15]. They are then detached by trypsinization and subcultured for many passages, giving long-term cultures. Using this method, comparable and reproducible populations of MSCs have been generated in many laboratories [4, 7, 26, 37].
Figure 1.
Mesenchymal stem cells in culture.
Transplantability and engraftment of MSCs
Numerous studies have demonstrated migration and multiorgan engraftment of MSCs both in animal models and in human clinical trials [43–48].
Direct injection of human marrow stromal cells into the corpus striatum of rat brain showed engraftment of 20% of the infused cells [48].
Injection of MSCs into the lateral ventricle of neonatal mice migrated throughout the forebrain and cerebellum [44]. Rat bone marrow stromal cells infused distally into areas of occluded ascending aorta migrated after eight weeks into the scar and periscar tissue [47].
MSCs injected intravenously into irradiated primates could engraft in different injured tissues, such as bone marrow, skin, digestive tract, and muscle [49, 50]. MSCs infused into mice homed into thymus [46].
In rat models, rat MSC have been engrafted in multiple organs, such as lung, liver, kidney and spleen. However, homing of labeled MSCs to the marrow of long bones was significantly increased by pre-treatment with vasodilators [51].
Human MSCs engrafted into sheep [52, 53] or mouse [54–56] show site-specific differentiation. The ability of MSC to engraft was influenced neither by the route of adminstration nor by the difference in conditioning protocols [57].
Both autologous and allogeneic MSCs have been given to patients [25, 58, 59]. Allogeneic HLA-mismatched male foetal cells injected into HLA-incompatible female fetal cells with osteogenesis imperfecta (OI) engrafted and differentiated into bone [60]. Haploidentical MSCs had a low level of engraftment in a patient with aplastic anemia, but there was a partial restoration of the bone marrow microenvironment [61]. In contrast, infused allogeneic MSCs did not expand substantially in patients. [59, 62].
Role of MSCs in support of hematopoiesis
MSCs support medullary hematopoiesis structurally and functionally by providing growth factors and extracellular matrix [63–67, 42].
Co-transplantation of HSCs along with MSCs ameliorated hematopoietic reconstitution [25, 68, 69]. MSCs maintain the expansion of lineage, specific colony-forming units of marrow CD34+ HSC. MSCs enhance engraftment of dose limited allogeneic and umbilical cord-derived HSC in NOD/SCID and in fetal sheep [70–72]. This promoting effect of MSCs was present even though MSCs were not detected in the BM of the host [73].
Co-transplantation of human MSCs enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice [72] and increases the functional hematopoietic microenvironment [74]. In humans, rapid hematopoietic recovery was demonstrated after co-infusion of autologous-blood stem cells and culture-expanded MSCs in advanced breast cancer patients receiving high-dose chemotherapy [23].
Co-transplantation of HLA-identical (sibling) culture-expanded MSCs with an HLA-identical (sibling) HSC transplant induced hematopoietic recovery on peripheral mononuclear cells (PMNC) and platelets [24, 75].
MSC plasticity, differentiation, and prospective use in regenerative medicine and treatment of various diseases
Human MSCs are multipotent and easily expanded. They represent potential clinical tools for tissue repair and gene therapy.
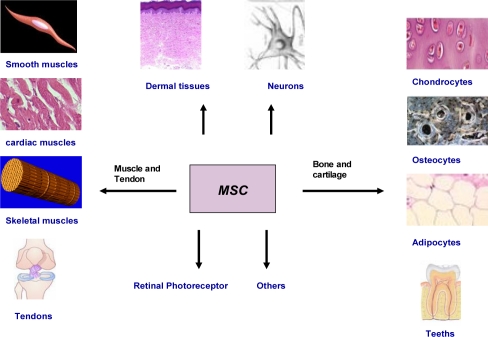
MSCs have a plastic potential. Plasticity means the ability of cells to convert from one type to another, which is also known as horizontal progression (synonomous to differentiation). In vitro and in vivo studies have indicated the ability of MSCs to differentiate into muscle, neural precursors, myocardial tissues, cardiomyocytes, bone, tendon, cartilage, and possibly other cell types (Figure 2). MSCs also produce appreciable amounts of lysosomal enzyme activity, which could correct metabolic derangements when given to enzyme-deficient patients with lysosomal storage diseases and other neurometabolic illnesses [76].
Figure 2.
Different possible cells that could be obtained from MSCs
Here we will outline briefly the results of several studies demonstrating the ability of MSC to differentiate into different tissues.
Muscle and heart
Repeated endomyocardial transplantation of high doses of allogeneic MSCs appeared safe in Yorkshire swine models [77]. Adult human MSCs showed persistent engraftment into infarcted rat myocardium [78]. MSCs enhanced the survival of existing myocytes in mice through paracrine mechanisms [79]. In murine models, single clonally purified MSCs seem to be more beneficial than unpurified transplantated MSCs in cardiac repair [80].
Transplantation of MSC combined with treatment with erythropoietin in rat models of acute myocardial infarction leads to enhancement of capillary density, and reduction of infarct size and fibrotic areas, as compared to groups that received only MSCs [81]. Transplantation of genetically engineered MSCs expressing an anti-apoptotic and angiogenic peptide improved cardiac function after myocardial infarction significantly more than MSCs alone [82]. MSCs implanted in a rat myocardial infarct heart improved cardiac structure and function through the combined effect of myogenesis and angiogenesis [83]. Fischer rats transplanted with MSCs transduced with an adenovirus expressing the Ang-1 and Akt genes were more resistant to anoxia and restored global cardiac function [84]. However, MSC proliferation in vitro was inhibited by aspirin, which is used extensively to treat cardiovascular diseases [85].
MSCs can differentiate into smooth muscles in rat models [86] and skeletal muscles in rat and mouse models, respectively [87, 88]. Human fetal MSCs transplanted into the uterus of mice with Duchenne muscular dystrophy distributed widely and differentiated into muscle cells. However, this did not cure the disease [89].
Nervous and renal system
Implantation of MSCs into injured spinal cords of rhesus monkeys elicited de novo neurogenesis and promoted functional recovery, as determined by tests of cortical somatosensory-evoked potential (CSEP) and motor-evoked potential (MEP). This also led to nearly normal sensory responses three months after transplantation [90]. Following spinal cord injury (SCI), MSCs had a positive effect on behavioural outcomes and histopathological assessments. They induced better recovery of hind limb sensitivity and increased the spared white matter in rat models [91]. In mice, transdifferentiated MSCs implanted into devitalised muscle grafts could support peripheral nerve regeneration to some extent [92]. Intrastriatal transplantation of MSCs promoted functional improvement on the rotarod test in murine models of Parkinson's disease [93]. Addition of MSCs to degenerative disc cells with anulus fibrosis (AF) in vitro resulted in changes in extracellular matrix biosynthesis with an upregulation of proteoglycan synthesis [94].
In murine models of experimental autoimmune encephalomyelitis, administration of MSCs at onset and at the peak of disease decreased inflammatory infiltrates and demyelination in the central nervous system [95]. In rhesus monkeys, implantation of a cellular allogenic nerve grafts and autologous MSCs repaired extended peripheral nerve lesions [96].
In humans, MSCs transdifferentiated into neural stem cells and improved the electrical and functional recovery of two patients with chronic spinal injury [97]. MSC infusion into patients suffering from metachromatic leukodystrophy and Hurler Syndrome was associated with significant improvement in nerve conduction velocities [25].
Transplanted MSCs accelerated glomerular healing in experimental rat models with glomerulonephritis [98]. In mice, MSCs reduced interstitial fibrosis, but did not delay progression of chronic kidney disease [99]. MSCs may protect against acute renal injury and promote the recovery of morphological and functional alterations of tubular epithelial cells [54]. In murine models, MSCs improved tissue damage triggered by renal ischemia and reperfusion injury [100].
Skin and related tissue
Human MSCs derived from the early human embryo can transform into epidermal cells in vitro and in vivo [101]. Injection of autologous biograft composed of autologous skin fibroblasts on biodegradable collagen membrane (Coladerm) in combination with autologous MSCs into the edges of the wound decreased wound size and increased the vascularity of the dermis of diabetic foot wounds [102]. Infusion of MSCs promoted the survival of allogeneic skin grafts in mice [103] and baboons [104].
Injection of human MSCs derived from umbilical cord blood into four men with Buerger's disease relieved ischemic rest pain in their affected extremities, led to healing of necrotic skin lesions within four weeks, and improved peripheral circulation [105].
Bone, cartilage and tendons
MSCs expanded in an osteoconductive carrier regenerated a critical segmental defect in the femur of dogs as effectively as autogenous cancellous bone. Mismatched allogeneic stem cells regenerated bone without eliciting an immunologic response. This finding raised the possibility of establishing allogeneic MSC banks for bone regeneration [106]. MSCs were able to reconstitute different layers in the femoral condyle [107]. In a canine model, transplantation of MSCs with partially demineralized bone matrix restored bone defects and enhanced bone growth [108]. In a rabbit model, MSCs regenerated full-thickness defects of articular cartilage defects, repaired Achilles tendon [107, 109], and helped to strengthen osteoporotic bone [110]. Implantation of rat demineralised bone matrices (DBM) with MSCs led to the formation of bone [111]. MSCs engrafted in mice with OI led to a significant increase in bone collagen and mineral content [112]. In an ovine model, spraying autologous MSCs onto grooved hydroxyapatite-coated collars of segmental bone tumor implants increased bone growth [113]. However, naive MSCs injected in mouse knee joints could not differentiate to restore cartilage tissue [114]. In addition, MSCs transplanted to ectopic sites in mice underwent alterations related to endochondral ossification rather than adopting a stable chondrogenic phenotype [115].
Use of MSCs in five children with OI disease lead to a signifincat increase in the total body mineral content and increased growth velocity [116, 117]. Three-dimensional tissue scaffolds promoted MSC ectopic bone formation [118]. MSCs that were used to fill bone defects during revision total joint replacement survived normal impact force during this procedure [119].
Retina tissues, liver and teeth
MSCs formed structures similar to the photoreceptor layer and expressed a photoreceptor-specific marker in rats [120], and could provide a beneficial effect in retinitis pigmentosa [121]. Murine MSCs integrated into retinal pigment displayed neuronal and glial morphologies and preserved photoreceptor cells in the rhodopsin knockout mouse [121].
Human MSCs grown in vitro gain the characteristic morphology and function of hepatocytes after transplantation into livers of immunodeficient mice; they engrafted and retained function of hepatocytes [122].
In vitro and in vivo studies demonstrated that MSCs can differentiate into functional odontoblast-like cells [123]. New populations of stem cells isolated from the root papilla of human teeth transplanted with periodontal ligament stem cells (PDLSCs) generated a root/periodontal complex capable of supporting a porcelain crown and resulting in normal tooth strength and appearance [124].
Role of MSCs in immunomodulation
Coculture of MSCs with allogeneic lymphocytes failed to stimulate their proliferation, indicating that these cells are innately not immunogeneic [125, 126, 104]. Recent reports suggest that MSCs have immunomodulatory properties and can inhibit lymphocyte antigen presenting cells, natural killer cells, and cytotoxic lymphocyte proliferation in mixed-lymphocyte reactions (MLR) [27, 30, 70, 104, 125–128].
MSCs inhibit CD2, CD4 and CD8 subsets of T lymphocytes [127, 128]. Despite the expression of HLA by MSCs, they were well tolerated without side effects in allogeneic hosts [27, 61, 104, 129].
Reports on the underlying mechanisms of MSC-mediated inhibitory effects are contradictory. Soluble inhibitory factors, such as hepatocyte growth factor [127], transforming growth factor-B [127], indoleamine oxidase [130], human leukocyte antigen-G [131] and interleukin-10 [132] have been implicated as mediators of the MSC inhibtory effect. However, the implication of TGFB, interleukin-10 and indoleamine oxidase has not been demonstrated by others [27].
The importance of cellular contact between MSCs and lymphocytes in enhancement of MSCs inhibtory effect is contradictory [127, 132, 133].
Co-transplantation of MSCs may prevent lethal graft-versus-host disease (GVHD) in MHC-mismatched murine HSC transplantation [129]. In a baboon model, MSC injection led to prolonged skin allograft survival [104]. Intravenous administration of MSCs prolonged the survival of transplanted hearts [134].
In humans, MSCs were used to treat severe acute GVHD [135]. MSCs derived from autoimmune disease (AD) patients exhibited extensive anti-proliferative properties against lymphocytes in vitro. This could be investigated as a form of immunomodulatory cellular therapy for AD patients [136].
In contrast, allogeneic and transgeneic MSCs were rejected by mismatched recipient mice [137, 138]. In addition, concurrent treatment with low-dose cyclosporine A and MSCs accelerated allograft rejection [139]. MSCs failed to prevent acute GVHD in mice [140].
MSCs and solid organ graft
MSCs revitalized cryopreserved allogeneic grafts used to repair large musculoskeletal defects [141]. They incorporated within the tissue sheath around the tendon, and adopted the characteristic spindle-shaped morphology of tenocyte-like cells [141]. MSC transplantation into heart enhanced cell survival, improved angiomyogenesis, and restored global cardiac function [84]. The vascular protheses, the inner surfaces of which are covered with MSCs that overexpress nitric oxide synthase, may have longer graft patency and vasculoprotective effects [142]. Injection of MSC enhanced xenochimerism in murine models, thereby showing promise as a strategy to achieve whole organ xenograft tolerance [143].
Role of MSCs in irradiation injury and in burns
Exposure of living cells to irradiation induces DNA damage and results in immediate tissue aplasia, or long term secondary effects resulting in induction of cancer [144, 145]. Acute radiation syndrome affects hematopoietic, gastrointestinal, neurovascular and cutanous systems [146]. Therapeutic irradiation can induce a significant decrease of both mature and immature progenitors in human BM and peripheral blood immediately after low-dose total body irradiation (TBI) (147). A dose of 2-8 Gy causes the hematopoietic component of the acute radiation syndrome in humans [148–150]. It has been demonstrated that growth of irradiated CD34+ cells was enhanced by co-culture with MSCs [151]. Injection of MSCs could help in the management of therapeutic irradiation side effects. Radiation enteritis is a functional disorder of the intestine that can occur during or after a course of radiotherapy of the abdomen, pelvis or rectum.
Radiation enteritis can present either as an acute or a chronic form, both of which have life threatening sequelae. The increasing use of radiotherapy in the treatment of solid organ malignancies in the abdomen and pelvis is likely to increase the incidence of radiation enteropathy in the future [152]. Moreover, it can damage normal tissues during the course of therapy for a few weeks after therapy, or even for months or years [153].
The first challenge in therapeutic MSC transplantation is how to efficiently deliver it to the sites of intended action. TBI increased human MSC engrafment in BM and muscle and further led to engraftment in brain, heart and liver [50]. Local irradiation in addition to TBI induces homing of human MSCs to exposed sites and promotes widespread engraftment to multiple organs in murine models [50]. It seems that inflammation and tissue injury due to irradiation activate molecular pathways that increase the release of tissue chemokines. This attracts MSCs to injured tissue, where they engraft and differentiate into different tissues to replace the injured areas and repair damage. MSCs accelerate structural recovery and favour healing of irradiated tissues [154]. Human MSCs were shown to support the structural regeneration of the small intestine in NOD/SCID mice after abdominal irradiation [154]. Amazingly, MSCs are resistant to irradiation [155]. Cells expressing the MSC phenotype were more prevalent in the peripheral blood of burn patients than in healthy donors. The percentage of MSCs correlated with the size and severity of burns, and with patient age [156]. Human MSCs favour healing of cutaneous radiation syndrome in a xenogenic transplant model [157].
MSCs in gene therapy
Transplantation of interleukin-7 (IL-7) gene-engineered MSCs into lethally irradiated mice led to a significant increase in thymopoiesis and homeostatic expansion of peripheral T lymphocytes. It also protected the host from GVHD and enhanced immune reconstitution [158].
In a murine model, MSCs transfected ex vivo with the hepatocyte growth factor gene were more therapeutically efficient than MSCs alone in protecting brain tissues from acute ischemic damage in the midcerebral artery occlusion [159].
Transduction with the brain-derived neurotrophic factor gene further enhanced the protective efficacy against ischemic damage [160].
Hypoxia-regulated HO-1 vector modification of MSCs enhanced the tolerance of engrafted MSCs to hypoxia-reoxygen injury in vitro and improved their viability in ischemic hearts [161].
MSCs in cancer
MSCs possess excellent migratory ability and exert inhibitory effects on the proliferation of glioma cells [162]. Modification of MSCs by gene therapy with therapeutic cytokines augments the anti-tumor effect and prolongs the survival of tumor-bearing animals [163]. MSCs transfected with the epidermal growth factor receptor exhibit enhanced therapeutic potential against murine brain tumors [164].
In a model of Kaposi's sarcoma, human MSCs injected intravenously homed to sites of tumorigenesis and potently inhibited tumor growth [165]. In a murine model, MSCs adenovirally-engineered to secrete interleukin-12 prevented revival and recurrence of tumor cells, which had escaped from conventional treatment [166].
MSC-engineered hydroxyapatite used to fill the patient's bone cavity after tumor curettage demonstrated healing potential without adverse reactions [167].
Genetically modified MSCs expressing the vascular endothelial growth factor receptor (tsFlk1) gene can inhibit growth of Burkitt's lymphoma in a murine model [168]. MSCs can target tumour cells [162] and have been suggested as a possible approach for the delivery of therapeutic agents [169]. MSCs transduced with an adenoviral expression vector carrying the human IFN-beta gene suppressed the growth of pulmonary metastases, presumably through the local production of IFN-beta in the tumor microenvironment [170].
By contrast, it has been demonstrated that MSCs could favour tumour growth in murine models [126, 171], but they do not interfere with the kinetics of tumor development [171]. MSCs recruit primary follicular lymphoma (FL) cells and trigger their differentiation into fibroblastic reticular cells, making them able to support malignant B-cell survival [172].
MSCs target microscopic tumors and contribute to the formation of a significant portion of tumor stroma development in vivo [173].
Tumor cells, when mixed with MSCs and transplanted subcutaneously, exhibited increased capability of proliferation and angiogenesis in tumour tissues and highly metastatic ability. When human marrow-derived MSCs were injected into tail veins of SCID mice bearing human malignant melanoma, human cells incorporated into tumor vessels and participated in angiogenesis [174]. Interaction of Multiple Myeloma cells with MSCs resulted in the formation and persistence of osteolytic bone lesions. However, 6-bromoindirubin-3'-monoxime treatment reduces the MSCs-stimulated proliferation of Multiple Myeloma cells and may enable MSCs to repair existing osteolytic lesions [175].
These results are contradictory, and further experimental and clinical studies are needed to evaluate the benificial effects of MSCs in cancer therapy.
Conclusion
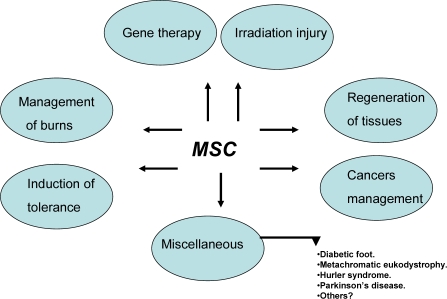
MSCs have a multipotent capacity. They support hematopoiesis and have immunomodulatory activity. Experimental and clinical studies have implicated MSCs in tissue repair. These characteristics make MSCs particularly attractive for therapeutic exploitation, such as regeneration of various tissues, induction of tolerance in solid organ graft, and BM transplantation. Figure 3 summarizes the possible therapeutic applications of MSCs. However, the beneficial versus deleterious effects of MSCs remain controversial. For instance, some studies showed the tolerogeneic effect of MSCs in recipients, while others showed that MSCs tended to promote rejection. Some reports demonstrated that MSCs had favourable effects on tumour growth, while others found that MSCs reduced the delay for tumour occurrence.
Figure 3.
Possible future therapeutic application of MSCs.
MSCs could provide opportunities for future clinical use in cellular therapy. However, more studies are needed on engraftment capacity, differentiation, and possible adverse effects in vivo.
References
- 1.Urist MR, Mc LF. Osteogenetic potency and new-bone formation by induction in transplants to the anterior chamber of the eye. J Bone Joint Surg Am. 1952;34-A(2):443–476. [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401(6751):390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 9.McKay R. Stem cells in the central nervous system. Science. 1997;276(5309):66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 10.Bach SP, Renehan AG, Potten CS. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis. 2000;21(3):469–476. doi: 10.1093/carcin/21.3.469. [DOI] [PubMed] [Google Scholar]
- 11.van Dorp AG, Verhoeven MC, Nat-Van Der Meij TH, Koerten HK, Ponec M. A modified culture system for epidermal cells for grafting purposes: an in vitro and in vivo study. Wound Repair Regen. 1999;7(4):214–225. doi: 10.1046/j.1524-475x.1999.00214.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 13.Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46(12):3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 14.Devine SM. Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem Suppl. 2002;38:73–79. doi: 10.1002/jcb.10046. [DOI] [PubMed] [Google Scholar]
- 15.Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9(5):747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28(8):875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 17.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64(2):278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107(2):275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 19.Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3(5):393–396. doi: 10.1080/146532401753277229. [DOI] [PubMed] [Google Scholar]
- 20.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20(6):530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 21.Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13(1):81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 23.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11(5):389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30(4):215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 26.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13(1):69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 27.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 28.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 29.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10(2):228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 30.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171(7):3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 31.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 32.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176(1):57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells fromhuman adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 34.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 35.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 36.Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, et al. Isolation of mesenchymal stem cells from GCSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37(10):967–976. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 37.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 38.Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92(11):4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plasticadherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97(7):3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meuleman N, Tondreau T, Delforge A, Dejeneffe M, Massy M, Libertalis M, et al. Human marrow mesenchymal stem cell culture: serum-free medium allows better expansion than classical alpha-MEM medium. Eur J Haematol. 2006;76(4):309–316. doi: 10.1111/j.1600-0609.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 41.Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205(2):228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 42.Gregory CA, Singh H, Perry AS, Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278(30):28067–28078. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]
- 43.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95(7):3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101(8):2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Hisha H, Inaba M, Lian Z, Yu C, Kawamura M, et al. A role of stromal cells in positive selection. Exp Hematol. 2000;28(8):950–960. doi: 10.1016/s0301-472x(00)00483-5. [DOI] [PubMed] [Google Scholar]
- 47.Saito T, Kuang JQ, Lin CC, Chiu RC. Transcoronary implantation of bone marrow stromal cells ameliorates cardiac function after myocardial infarction. J Thorac Cardiovasc Surg. 2003;126(1):114–123. doi: 10.1016/s0022-5223(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 48.Phinney DG, Baddoo M, Dutreil M, Gaupp D, Lai WT, Isakova IA. Murine mesenchymal stem cells transplanted to the central nervous system of neonatal versus adult mice exhibit distinct engraftment kinetics and express receptors that guide neuronal cell migration. Stem Cells Dev. 2006;15(3):437–447. doi: 10.1089/scd.2006.15.437. [DOI] [PubMed] [Google Scholar]
- 49.Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5(12):1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 50.Francois S, Bensidhoum M, Mouiseddine M, Mazurier C, Allenet B, Semont A, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24(4):1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 51.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 52.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 53.Airey JA, Almeida-Porada G, Colletti EJ, Porada CD, Chamberlain J, Movsesian M, et al. Human mesenchymal stem cells form Purkinje fibers in fetal sheep heart. Circulation. 2004;109(11):1401–1407. doi: 10.1161/01.CIR.0000124222.16321.26. [DOI] [PubMed] [Google Scholar]
- 54.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14(6):1035–1041. [PubMed] [Google Scholar]
- 55.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 57.Mahmud N, Pang W, Cobbs C, Alur P, Borneman J, Dodds R, et al. Studies of the route of administration and role of conditioning with radiation on unrelated allogeneic mismatched mesenchymal stem cell engraftment in a nonhuman primate model. Exp Hematol. 2004;32(5):494–501. doi: 10.1016/j.exphem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557–564. [PubMed] [Google Scholar]
- 59.Wang J, Liu K, Lu DP. Mesenchymal stem cells in stem cell transplant recipients are damaged and remain of host origin. Int J Hematol. 2005;82(2):152–158. doi: 10.1532/IJH97.A10505. [DOI] [PubMed] [Google Scholar]
- 60.Le Blanc K, Gotherstrom C, Ringden O, Hassan M, McMahon R, Horwitz E, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79(11):1607–1614. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 61.Fouillard L, Bensidhoum M, Bories D, Bonte H, Lopez M, Moseley AM, et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17(2):474–476. doi: 10.1038/sj.leu.2402786. [DOI] [PubMed] [Google Scholar]
- 62.Dickhut A, Schwerdtfeger R, Kuklick L, Ritter M, Thiede C, Neubauer A, et al. Mesenchymal stem cells obtained after bone marrow transplantation or peripheral blood stem cell transplantation originate from host tissue. Ann Hematol. 2005;84(11):722–727. doi: 10.1007/s00277-005-1067-8. [DOI] [PubMed] [Google Scholar]
- 63.Suda T, Arai F, Shimmura S. Regulation of stem cells in the niche. Cornea. 2005;24(8 Suppl):S12–S17. doi: 10.1097/01.ico.0000178742.98716.65. [DOI] [PubMed] [Google Scholar]
- 64.Heissig B, Ohki Y, Sato Y, Rafii S, Werb Z, Hattori K. A role for niches in hematopoietic cell development. Hematology. 2005;10(3):247–253. doi: 10.1080/10245330500067249. [DOI] [PubMed] [Google Scholar]
- 65.Arai F, Hirao A, Suda T. Regulation of hematopoietic stem cells by the niche. Trends Cardiovasc Med. 2005;15(2):75–79. doi: 10.1016/j.tcm.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Verfaillie CM. Adhesion receptors as regulators of the hematopoietic process. Blood. 1998;92(8):2609–2612. [PubMed] [Google Scholar]
- 67.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity. 1999;11(5):555–566. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- 68.Li N, Feugier P, Serrurrier B, Latger-Cannard V, Lesesve JF, Stoltz JF, et al. Human mesenchymal stem cells improve ex vivo expansion of adult human CD34+ peripheral blood progenitor cells and decrease their allostimulatory capacity. Exp Hematol. 2007;35(3):507–515. doi: 10.1016/j.exphem.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 69.Han JY, Goh RY, Seo SY, Hwang TH, Kwon HC, Kim SH, et al. Cotransplantation of cord blood hematopoietic stem cells and culture-expanded and GM-CSF-/SCF-transfected mesenchymal stem cells in SCID mice. J Korean Med Sci. 2007;22(2):242–247. doi: 10.3346/jkms.2007.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33(6):597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 71.Almeida-Porada G, Flake AW, Glimp HA, Zanjani ED. Cotransplantation of stroma results in enhancement of engraftment and early expression of donor hematopoietic stem cells in utero. Exp Hematol. 1999;27(10):1569–1575. doi: 10.1016/s0301-472x(99)00090-9. [DOI] [PubMed] [Google Scholar]
- 72.Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L, et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 2003;31(5):413–420. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 73.Anker PS, Noort WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R, et al. Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2003;31(10):881–889. doi: 10.1016/s0301-472x(03)00202-9. in't . [DOI] [PubMed] [Google Scholar]
- 74.Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107(5):1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- 75.Fouillard L, Chapel A, Bories D, Bouchet S, Costa JM, Rouard H, et al. Infusion of allogeneic-related HLA mismatched mesenchymal stem cells for the treatment of incomplete engraftment following autologous haematopoietic stem cell transplantation. Leukemia. 2007 doi: 10.1038/sj.leu.2404550. [DOI] [PubMed] [Google Scholar]
- 76.Muller I, Kustermann-Kuhn B, Holzwarth C, Isensee G, Vaegler M, Harzer K, et al. In vitro analysis of multipotent mesenchymal stromal cells as potential cellular therapeutics in neurometabolic diseases in pediatric patients. Exp Hematol. 2006;34(10):1413–1419. doi: 10.1016/j.exphem.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 77.Poh KK, Sperry E, Young RG, Freyman T, Barringhaus KG, Thompson CA. Repeated direct endomyocardial transplantation of allogeneicmesenchymal stem cells: Safety of a high dose, “off-the-shelf”,cellular cardiomyoplasty strategy. Int J Cardiol. 2007;117(3):360–364. doi: 10.1016/j.ijcard.2006.04.092. [DOI] [PubMed] [Google Scholar]
- 78.Grinnemo KH, Mansson A, Dellgren G, Klingberg D, Wardell E, Drvota V, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127(5):1293–1300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 79.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14(6):840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 80.Zhang S, Ge J, Sun A, Xu D, Qian J, Lin J, et al. Comparison of various kinds of bone marrow stem cells for the repair of infarcted myocardium: single clonally purified nonhematopoietic mesenchymal stem cells serve as a superior source. J Cell Biochem. 2006;99(4):1132–1147. doi: 10.1002/jcb.20949. [DOI] [PubMed] [Google Scholar]
- 81.Zhang D, Zhang F, Zhang Y, Gao X, Li C, Yang N, et al. Combining erythropoietin infusion with intramyocardial delivery of bone marrow cells is more effective for cardiac repair. Transpl Int. 2007;20(2):174–183. doi: 10.1111/j.1432-2277.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 82.Jo JI, Nagaya N, Miyahara Y, Kataoka M, HaradaShiba M, Kangawa K, et al. Transplantation of Genetically Engineered Mesenchymal Stem Cells Improves Cardiac Function in Rats With Myocardial Infarction: Benefit of a Novel Nonviral Vector, Cationized Dextran. Tissue Eng. 2007;13(2):313–22. doi: 10.1089/ten.2006.0133. [DOI] [PubMed] [Google Scholar]
- 83.Tang J, Xie Q, Pan G, Wang J, Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006;30(2):353–361. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 84.Jiang S, Haider H, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99(7):776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Chen X, Zhu W, Zhang H, Hu S, Cong X. Growth inhibition of mesenchymal stem cells by aspirin: involvement of the WNT/beta-catenin signal pathway. Clin Exp Pharmacol Physiol. 2006;33(8):696–701. doi: 10.1111/j.1440-1681.2006.04432.x. [DOI] [PubMed] [Google Scholar]
- 86.Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109(1):74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 87.Seruya M, Shah A, Pedrotty D, du Laney T, Melgiri R, McKee JA, et al. Clonal population of adult stem cells: life span and differentiation potential. Cell Transplant. 2004;13(2):93–101. doi: 10.3727/000000004773301762. [DOI] [PubMed] [Google Scholar]
- 88.Lee JH, Kosinski PA, Kemp DM. Contribution of human bone marrow stem cells to individual skeletal myotubes followed by myogenic gene activation. Exp Cell Res. 2005;307(1):174–182. doi: 10.1016/j.yexcr.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Chan J, Waddington SN, O'Donoghue K, Kurata H, Guillot PV, Gotherstrom C, et al. Widespread distribution and muscle differentiation of human fetal mesenchymal stem cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells. 2007;25(4):875–884. doi: 10.1634/stemcells.2006-0694. [DOI] [PubMed] [Google Scholar]
- 90.Deng YB, Liu XG, Liu ZG, Liu XL, Liu Y, Zhou GQ. Implantation of BM mesenchymal stem cells into injured spinal cord elicits de novo neurogenesis and functional recovery: evidence from a study in rhesus monkeys. Cytotherapy. 2006;8(3):210–214. doi: 10.1080/14653240600760808. [DOI] [PubMed] [Google Scholar]
- 91.Urdzikova L, Jendelova P, Glogarova K, Burian M, Hajek M, Sykova E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23(9):1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- 92.Keilhoff G, Goihl A, Stang F, Wolf G, Fansa H. Peripheral nerve tissue engineering: autologous Schwann cells vs. transdifferentiated mesenchymal stem cells. Tissue Eng. 2006;12(6):1451–1465. doi: 10.1089/ten.2006.12.1451. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neurosci Lett. 2001;316(2):67–70. doi: 10.1016/s0304-3940(01)02384-9. [DOI] [PubMed] [Google Scholar]
- 94.Le Visage C, Kim SW, Tateno K, Sieber AN, Kostuik JP, Leong KW. Interaction of human mesenchymal stem cells with disc cells: changes in extracellular matrix biosynthesis. Spine. 2006;31(18):2036–2042. doi: 10.1097/01.brs.0000231442.05245.87. [DOI] [PubMed] [Google Scholar]
- 95.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 96.Hu J, Zhu QT, Liu XL, Xu YB, Zhu JK. Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol. 2007;204(2):658–666. doi: 10.1016/j.expneurol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 97.Moviglia GA, Fernandez Vina R, Brizuela JA, Saslavsky J, Vrsalovic F, Varela G, et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy. 2006;8(3):202–209. doi: 10.1080/14653240600736048. [DOI] [PubMed] [Google Scholar]
- 98.Kunter U, Rong S, Djuric Z, Boor P, Muller-Newen G, Yu D, et al. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17(8):2202–2212. doi: 10.1681/ASN.2005080815. [DOI] [PubMed] [Google Scholar]
- 99.Ninichuk V, Gross O, Segerer S, Hoffmann R, Radomska E, Buchstaller A, et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70(1):121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 100.Semedo P, Wang PM, Andreucci TH, Cenedeze MA, Teixeira VP, Reis MA, et al. Mesenchymal stem cells ameliorate tissue damages triggered by renal ischemia and reperfusion injury. Transplant Proc. 2007;39(2):421–423. doi: 10.1016/j.transproceed.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 101.Wu M, Yang L, Liu S, Li H, Hui N, Wang F, et al. Differentiation potential of human embryonic mesenchymal stem cells for skin-related tissue. Br J Dermatol. 2006;155(2):282–291. doi: 10.1111/j.1365-2133.2006.07357.x. [DOI] [PubMed] [Google Scholar]
- 102.Vojtassak J, Danisovic L, Kubes M, Bakos D, Jarabek L, Ulicna M, et al. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol Lett. 2006;27(Suppl 2):134–137. [PubMed] [Google Scholar]
- 103.Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17(3):240–248. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 104.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 105.Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, et al. Successful stem cell therapy using umbilical cord bloodderived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006;24(6):1620–1626. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 106.Kraus KH, Kirker-Head C. Mesenchymal stem cells and bone regeneration. Vet Surg. 2006;35(3):232–242. doi: 10.1111/j.1532-950X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 107.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76(4):579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 108.Shih HN, Shih LY, Sung TH, Chang YC. Restoration of bone defect and enhancement of bone ingrowth using partially demineralized bone matrix and marrow stromal cells. J Orthop Res. 2005;23(6):1293–1299. doi: 10.1016/j.orthres.2005.04.005.1100230609. [DOI] [PubMed] [Google Scholar]
- 109.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16(4):406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 110.Wang Z, Goh J, Das De S, Ge Z, Ouyang H, Chong JS, Low SL, et al. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12(7):1753–1761. doi: 10.1089/ten.2006.12.1753. [DOI] [PubMed] [Google Scholar]
- 111.Krugliakov PV, Sokolova IB, Zin'kova NN, Viide SV, Cherednichenko NN, Kisliakova TV, et al. The influence of mesenchymal stem cells on bone tissue regeneration upon implantation of demineralized bone matrix. Tsitologiia. 2005;47(6):466–477. [PubMed] [Google Scholar]
- 112.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95(3):1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalia P, Blunn GW, Miller J, Bhalla A, Wiseman M, Coathup MJ. Do autologous mesenchymal stem cells augment bone growth and contact to massive bone tumor implants? Tissue Eng. 2006;12(6):1617–1626. doi: 10.1089/ten.2006.12.1617. [DOI] [PubMed] [Google Scholar]
- 114.Noel D, Gazit D, Bouquet C, Apparailly F, Bony C, Plence P, et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells. 2004;22(1):74–85. doi: 10.1634/stemcells.22-1-74. [DOI] [PubMed] [Google Scholar]
- 115.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 116.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 117.Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97(5):1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 118.Hosseinkhani H, Azzam T, Kobayashi H, Hiraoka Y, Shimokawa H, Domb AJ, et al. Bone Tissue Engineering Through a Combination of 3-Dimensional Tissue Engineered Scaffold and Transfected Mesenchymal Stem Cells. Tissue Eng. 2006 [Google Scholar]
- 119.Korda M, Blunn G, Phipps K, Rust P, Di Silvio L, Coathup M, et al. Can mesenchymal stem cells survive under normal impaction force in revision total hip replacements? Tissue Eng. 2006;12(3):625–630. doi: 10.1089/ten.2006.12.625. [DOI] [PubMed] [Google Scholar]
- 120.Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23(21):7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245(3):414–422. doi: 10.1007/s00417-006-0382-7. [DOI] [PubMed] [Google Scholar]
- 122.Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56(3):405–415. doi: 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li ZY, Chen L, Liu L, Lin YF, Li SW, Tian WD. Odontogenic potential of bone marrow mesenchymal stem cells. J Oral Maxillofac Surg. 2007;65(3):494–500. doi: 10.1016/j.joms.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 124.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in Swine. PLoS ONE. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 126.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 127.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 128.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60(3):307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 129.Chung NG, Jeong DC, Park SJ, Choi BO, Cho B, Kim HK, et al. Cotransplantation of marrow stromal cells may prevent lethal graft-versus-host disease in major histocompatibility complex mismatched murine hematopoietic stem cell transplantation. Int J Hematol. 2004;80(4):370–376. doi: 10.1532/ijh97.a30409. [DOI] [PubMed] [Google Scholar]
- 130.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 131.Nasef A, Mathieu N, Chapel A, frick J, Francois S, Mazurier C, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84(2):231–237. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- 132.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 133.Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, et al. Identification of IL-10 and TGF-β transcripts involved in inhibiting T Lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene expression. 2007;13(4-5):217–26. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou HP, Yi DH, Yu SQ, Sun GC, Cui Q, Zhu HL, et al. Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplant Proc. 2006;38(9):3046–3051. doi: 10.1016/j.transproceed.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 135.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 136.Bocelli-Tyndall C, Bracci L, Spagnoli G, Braccini A, Bouchenaki M, Ceredig R, et al. Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and autoimmune disease patients reduce the proliferation of autologousand allogeneic-stimulated lymphocytes in vitro. Rheumatology (Oxford) 2006 doi: 10.1093/rheumatology/kel267. [DOI] [PubMed] [Google Scholar]
- 137.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I and class II-mismatched recipient mice. Blood. 2005;106(13):4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 138.Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24(11):2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- 139.Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HJ, Geissler EK, et al. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81(11):1589–1595. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]
- 140.Sudres M, Norol F, Trenado A, Gregoire S, Charlotte F, Levacher B, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176(12):7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 141.Ouyang HW, Cao T, Zou XH, Heng BC, Wang LL, Song XH, et al. Mesenchymal stem cell sheets revitalize nonviable dense grafts: implications for repair of large-bone and tendon defects. Transplantation. 2006;82(2):170–174. doi: 10.1097/01.tp.0000226232.79106.72. [DOI] [PubMed] [Google Scholar]
- 142.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, et al. Synthetic vascular prosthesis impregnated with mesenchymal stem cells overexpressing endothelial nitric oxide synthase. Circulation. 2006;114(1 Suppl):I327–330. doi: 10.1161/CIRCULATIONAHA.105.001586. [DOI] [PubMed] [Google Scholar]
- 143.Moadsiri A, Polchert D, Genrich K, Napoles P, Reina E, Turian J, et al. Mesenchymal stem cells enhance xenochimerism in NK-depleted hosts. Surgery. 2006;140(2):315–321. doi: 10.1016/j.surg.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 144.Tubiana M, Arriagada R, Sarrazin D. Human cancer natural history, radiation induced immunodepression and postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1986;12(4):477–485. doi: 10.1016/0360-3016(86)90055-6. [DOI] [PubMed] [Google Scholar]
- 145.Schmidt-Ullrich RK, Dent P, Grant S, Mikkelsen RB, Valerie K. Signal transduction and cellular radiation responses. Radiat Res. 2000;153(3):245–257. doi: 10.1667/0033-7587(2000)153[0245:stacrr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 146.Anno GH, Baum SJ, Withers HR, Young RW. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5-30 Gy. Health Phys. 1989;56(6):821–838. doi: 10.1097/00004032-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 147.Belkacemi Y, Bouchet S, Frick J, Huchet A, Pene F, Aigueperse J, et al. Monitoring of residual hematopoiesis after total body irradiation in humans as a model for accidental x-ray exposure: dose-effect and failure of ex vivo expansion of residual stem cells in view of autografting. Int J Radiat Oncol Biol Phys. 2003;57(2):500–507. doi: 10.1016/s0360-3016(03)00596-0. [DOI] [PubMed] [Google Scholar]
- 148.Stickney DR, Dowding C, Garsd A, Ahlem C, Whitnall M, McKeon M, et al. 5-androstenediol stimulates multilineage hematopoiesis in rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol. 2006;6(11):1706–1713. doi: 10.1016/j.intimp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 149.Hirama T, Tanosaki S, Kandatsu S, Kuroiwa N, Kamada T, Tsuji H, et al. Initial medical management of patients severely irradiated in the Tokai-mura criticality accident. Br J Radiol. 2003;76(904):246–253. doi: 10.1259/bjr/82373369. [DOI] [PubMed] [Google Scholar]
- 150.Hirama T, Akashi M. Multi-organ involvement in the patient who survived the Tokai-mura criticality accident. BJR Suppl. 2005;27:17–20. [Google Scholar]
- 151.Mourcin F, Grenier N, Mayol JF, Lataillade JJ, Sotto JJ, Herodin F, et al. Mesenchymal stem cells support expansion of in vitro irradiated CD34(+) cells in the presence of SCF, FLT3 ligand, TPO and IL3: potential application to autologous cell therapy in accidentally irradiated victims. Radiat Res. 2005;164(1):1–9. doi: 10.1667/rr3384. [DOI] [PubMed] [Google Scholar]
- 152.Toomey DP, Cahill RA, Geraghty J, Thirion P. Radiation enteropathy. Ir Med J. 2006;99(7):215–217. [PubMed] [Google Scholar]
- 153.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4(9):529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 154.Semont A, Francois S, Mouiseddine M, Francois A, Sache A, Frick J, et al. Mesenchymal stem cells increase selfrenewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol. 2006;585:19–30. doi: 10.1007/978-0-387-34133-0_2. [DOI] [PubMed] [Google Scholar]
- 155.Chen MF, Lin CT, Chen WC, Yang CT, Chen CC, Liao SK, et al. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys. 2006;66(1):244–253. doi: 10.1016/j.ijrobp.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 156.Mansilla E, Marin GH, Drago H, Sturla F, Salas E, Gardiner C, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967–969. doi: 10.1016/j.transproceed.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 157.Francois S, Mouiseddine M, Mathieu N, Semont A, Monti P, Dudoignon N, et al. Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann Hematol. 2007;86(1):1–8. doi: 10.1007/s00277-006-0166-5. [DOI] [PubMed] [Google Scholar]
- 158.Li A, Zhang Q, Jiang J, Yuan G, Feng Y, Hao J, et al. Co-transplantation of bone marrow stromal cells transduced with IL-7 gene enhances immune reconstitution after allogeneic bone marrow transplantation in mice. Gene Ther. 2006;13(15):1178–1187. doi: 10.1038/sj.gt.3302741. [DOI] [PubMed] [Google Scholar]
- 159.Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, Miyazawa D, et al. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26(9):1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- 160.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11(1):96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 161.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46(7):1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 162.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 163.Hamada H, Kobune M, Nakamura K, Kawano Y, Kato K, Honmou O, et al. Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci. 2005;96(3):149–156. doi: 10.1111/j.1349-7006.2005.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sato H, Kuwashima N, Sakaida T, Hatano M, Dusak JE, Fellows-Mayle WK, et al. Epidermal growth factor receptortransfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005;12(9):757–768. doi: 10.1038/sj.cgt.7700827. [DOI] [PubMed] [Google Scholar]
- 165.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203(5):1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen XC, Wang R, Zhao X, Wei YQ, Hu M, Wang YS, et al. Prophylaxis against carcinogenesis in three kinds of unestablished tumor models via IL12-gene-engineered MSCs. Carcinogenesis. 2006;27(12):2434–2441. doi: 10.1093/carcin/bgl069. [DOI] [PubMed] [Google Scholar]
- 167.Morishita T, Honoki K, Ohgushi H, Kotobuki N, Matsushima A, Takakura Y. Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients' mesenchymal stem cells. Artif Organs. 2006;30(2):115–118. doi: 10.1111/j.1525-1594.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 168.Kyriakou CA, Yong KL, Benjamin R, Pizzey A, Dogan A, Singh N, et al. Human mesenchymal stem cells (hMSCs) expressing truncated soluble vascular endothelial growth factor receptor (tsFlk-1) following lentiviral-mediated gene transfer inhibit growth of Burkitt's lymphoma in a murine model. J Gene Med. 2006;8(3):253–264. doi: 10.1002/jgm.840. [DOI] [PubMed] [Google Scholar]
- 169.Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123(4):702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 170.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96(21):1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 171.Djouad F, Bony C, Apparailly F, Louis-Plence P, Jorgensen C, Noel D. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation. 2006;82(8):1060–1066. doi: 10.1097/01.tp.0000236098.13804.0b. [DOI] [PubMed] [Google Scholar]
- 172.Ame-Thomas P, Maby-El Hajjami H, Monvoisin C, Jean R, Monnier D, Caulet-Maugendre S, et al. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109(2):693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- 173.Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11(21):7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 174.Sun B, Zhang S, Ni C, Zhang D, Liu Y, Zhang W, et al. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev. 2005;14(3):292–298. doi: 10.1089/scd.2005.14.292. [DOI] [PubMed] [Google Scholar]
- 175.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24(4):986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]