A principal goal in neuroscience is to understand how neuronal populations across different brain regions process information from the vibrant world, rich in moment-to-moment variations of sight, smell, sound, touch, etc. Traditional inquiries examine how the evoked neuronal responses differ with stimuli (e.g., sound or touch). However, the brain detects stimuli reliably even when ambient (or background) conditions shift owing to external (e.g., light vs. dark) and/or internal (e.g., alert vs. sleepy) factors. Recent investigations have assessed whether evoked neuronal activity (ΔN) varies when the brain's operational (or baseline) state is altered, whereby including an independent measure of baseline (or spontaneous) neuronal activity (No) provides a total measure of neuronal activity for the perturbed state (N). In PNAS, Li et al. (1) demonstrate in the rat's olfactory bulb that total bulbar activity level reached upon odor exposure (i.e., N = ΔN + No)—measured by extracellular recordings—is independent of the bulb's baseline activity level. This result agrees well with previous observations from other cortical sensory systems, using a variety of neuroimaging techniques [e.g., functional MRI (fMRI), optical imaging, and electrophysiology (2–9)]. Taken together, these studies (1–9) imply that there may be inherent neuronal mechanisms to ensure a similar level of information transfer from sensory input, regardless of external/internal situations that may impact the brain's baseline state (10).
Li et al. (1) measured odor-induced bulbar activity under different baseline states. Whereas spontaneous neuronal activity in the two baseline states (achieved by varying anesthetic doses) differed by approximately twofold, nearly identical levels of total neuronal activity were reached upon the same odor exposure from each baseline state. This phenomenon was observed regardless of which bulbar layer (mitral, granular) the recordings were obtained from, the type of anesthetic (pentobarbital, chloral hydrate) that was used to achieve the baseline state, and the type (aldehyde, ketone, ester) and concentration (up to fivefold difference) of the odor that was exposed to the rat. Previous optical imaging and fMRI studies examined odor-induced bulbar activity patterns and how topologies and intensities vary with concentration (11, 12). For a given odor the topology of the bulbar activity pattern is largely independent of concentration, but the intensity of bulbar activity correlates positively with concentration. Because these studies were conducted with only one baseline state, results from Li et al. (1) extend the previous findings regarding the neuronal encoding of quality (with pattern topology) and concentration of odor (with pattern intensity) across different baseline states. If the baseline state variation studies of the olfactory bulb (1) are combined with similar studies of the olfactory cortex (13), we may deduce that, regardless of the baseline state, maintaining high-fidelity transmission of smell signals—from the olfactory epithelium, through the olfactory bulb, to the olfactory cortex—could be a system-level phenomenon. Indeed, numerous laboratories (1–9) using a variety of neuroimaging techniques (e.g., fMRI, optical imaging, electrophysiology), studying dissimilar primary sensory systems (e.g., somatosensory cortex, visual cortex, olfactory bulb), across various species (e.g., rat, cat, monkey, human), and under diverse experimental conditions (e.g., awake and anesthetized with volatile and nonvolatile agents) observe that the total neuronal activity reached upon stimulation is, to a first order, independent of the spontaneous (i.e., nonevoked) baseline state neuronal activity (10).
Relationship Between Neuroimaging Signal and Neuronal Activity
The ability to do quantitative and spatially resolved electrophysiology is restricted for studies of human brain function. Consequently, mapping of functional activity in the human brain uses methods like fMRI, which unfortunately provide an indirect measure of neuronal activity. However, the fMRI signal can be calibrated to represent the brain's energy consumption (14, 15). A link between the results of Li et al. (1) and fMRI can be made on the basis of linear relationships that have been established between neuronal activity and energy these activities demand (16).
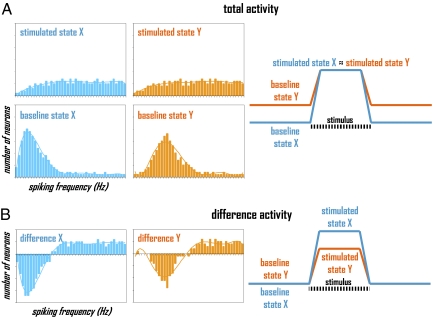
Extracellular recordings can be quantified in terms of spiking rate (or frequency) of many neurons (2, 4). A descriptive example, as shown in Fig. 1A and which is comparable to data presented by Li et al. (1), illustrates behavior of a neuronal population (Fig. 1A, Left) as well as its dynamic pattern (Fig. 1A, Right) when stimulated with the same stimulus, but from different baselines. Comparison of the histograms (composed of number of neurons firing at their respective signaling speeds) for the two baseline states shows that in state X the fraction of population at lower frequencies makes a greater contribution to the population's vote than in state Y (i.e., baseline state X < baseline state Y). However, the histograms for the two stimulated states have similar distributions (i.e., stimulated state X ≈ stimulated state Y), reached by redeployment (not recruitment) of a fraction of neuronal population. Because each histogram depicts the same neuronal population (Fig. 1A, Left), dynamics of the total activity (i.e., N = ΔN + No) shows that the neuronal activities in the two baseline states differ, but upon stimulation the neuronal activities reach the same levels (Fig. 1A, Right).
Fig. 1.
Neuronal activity for baseline and stimulated states, X and Y, represented in terms of neuronal population (histograms, Left) and dynamic pattern (time-courses, Right) when represented in terms of (A) total and (B) difference activity, respectively.
Estimates of relative neuronal energy demanded in each baseline and stimulated state shown in Fig. 1A can be obtained by integrating each histogram using a linear relationship (4) between oxidative energy demand (E) and the number of cells (Ni) firing at a given rate (vi) given scaling factors for neurons (G). For different brain states, relative total neuronal energy calculated from experimentally measured histograms (i.e., E = G ∑ Nivi) agrees quite well with oxidative energy demand measured by techniques like calibrated fMRI and 13C or 17O magnetic resonance spectroscopy (MRS) (14, 17, 18). However, careful considerations are necessary when histogram distributions span high and low frequencies in disproportionate fractions (4).
Should the “Evoked” or the “Total” Signal Be Measured in Neuroimaging?
Neuroscientists typically use fMRI (or PET) with task-based paradigms in which the mean signal of an undetermined baseline state is subtracted from the mean signal of the stimulated state to expose activated (or deactivated) regions associated with the task (10). Traditionally, subtracting the baseline signal was justified by an assumption that there was very little baseline neuronal activity (and by inference, demanded energy) in the awake state (10). However, recent methods—including 13C or 17O MRS and PET—have shown this hypothesis to be incorrect (15, 17–20). In the awake human brain, in fact, the total neuronal activity (or energy) in the baseline state is much higher than the incremental activity (or energy) evoked by stimuli (19). Thus, the subtraction-based method, as shown in Fig. 1B, leads to very different interpretation when baseline neuronal activity is subtracted from the activity during stimulation. Even though the final histograms upon stimulation are identical for states X and Y (Fig. 1A, Left), because the two baseline states have different distributions (Fig. 1A, Left), the subtracted histograms are quite dissimilar for states X and Y (Fig. 1B, Left). Therefore, if Li et al. (1) subtracted the baseline neuronal activity measured during odor stimulation (Fig. 1B, Right), they would arrive at the opposite conclusion, that the neuronal activity during odor simulation is baseline dependent. Overall, the comparison of total and difference activities (Fig. 1) shows the danger of not measuring the total neuronal activity in neuroimaging.
Conclusions
Because many vital functions (e.g., foraging, mating, survival, etc.) depend on the brain's ability to perceive the dynamically varying environment, neuronal systems need to accurately detect and process external stimuli under widely different operational circumstances (10). Indeed, an echoing principle in the Li et al. study (1) and others like it (2–9) is that the total neuronal activity in any given state is relevant for understanding brain function, from resting to even during stimulation (21, 22). Future research should test to what degree the cerebral cortex, which has very different neuronal composition than the olfactory bulb (23), demonstrates the same phenomenon. Despite the fact both awake humans (8, 9) and anesthetized animals (1–7) demonstrate this phenomenon, how anesthetics (which are used to vary the baseline) affect various brain regions should be studied more specifically, because for example, thalamic structures that innervate the cerebral cortex but not the olfactory bulb (23) are directly affected by anesthesia (20). In summary, it is crucial for neuroscientists to err on the side of baseline neuronal activity (or energy) and account for it in absolute terms by using methods like calibrated fMRI and others (14–20) so as to improve quantitative neuroimaging of brain function. A vital question, however, is whether it is the total neuronal activity (or energy) during task (N) or the difference from rest (ΔN = N – No) that is most relevant for understanding brain function (10).
Acknowledgments
The authors thank colleagues at University of Minnesota and Yale University, and are supported by National Institutes of Health Grants R01 MH-067528, R01 AG-034953, and P30 NS-052519.
Footnotes
The authors declare no conflict of interest.
See companion article on page 5087 in issue 12 of volume 108.
References
- 1.Li A, Gong L, Xu F. Brain-state–independent neural representation of peripheral stimulation in rat olfactory bulb. Proc Natl Acad Sci USA. 2011;108:5087–5092. doi: 10.1073/pnas.1013814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AJ, et al. Cerebral energetics and spiking frequency: The neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen LM, Friedman RM, Roe AW. Optical imaging of SI topography in anesthetized and awake squirrel monkeys. J Neurosci. 2005;25:7648–7659. doi: 10.1523/JNEUROSCI.1990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maandag NJ, et al. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci USA. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudetz AG, Imas OA. Burst activation of the cerebral cortex by flash stimuli during isoflurane anesthesia in rats. Anesthesiology. 2007;107:983–991. doi: 10.1097/01.anes.0000291471.80659.55. [DOI] [PubMed] [Google Scholar]
- 6.Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- 7.Zhu XH, Zhang N, Zhang Y, Uğurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab. 2009;29:10–18. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uludağ K, et al. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage. 2004;23:148–155. doi: 10.1016/j.neuroimage.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36:269–276. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: Implications for the interpretation of fMRI. Proc Natl Acad Sci USA. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wachowiak M, Cohen LB, Zochowski MR. Distributed and concentration-invariant spatial representations of odorants by receptor neuron input to the turtle olfactory bulb. J Neurophysiol. 2002;87:1035–1045. doi: 10.1152/jn.00522.2001. [DOI] [PubMed] [Google Scholar]
- 12.Xu F, Kida I, Hyder F, Shulman RG. Assessment and discrimination of odor stimuli in rat olfactory bulb by dynamic functional MRI. Proc Natl Acad Sci USA. 2000;97:10601–10606. doi: 10.1073/pnas.180321397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami M, Kashiwadani H, Kirino Y, Mori K. State-dependent sensory gating in olfactory cortex. Neuron. 2005;46:285–296. doi: 10.1016/j.neuron.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Hyder F, et al. Quantitative functional imaging of the brain: Towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- 15.Hoge RD, et al. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanganahalli BG, Herman P, Blumenfeld H, Hyder F. Oxidative neuroenergetics in event-related paradigms. J Neurosci. 2009;29:1707–1718. doi: 10.1523/JNEUROSCI.5549-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyder F, et al. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 18.Zhu XH, et al. Advanced in vivo heteronuclear MRS approaches for studying brain bioenergetics driven by mitochondria. Methods Mol Biol. 2009;489:317–357. doi: 10.1007/978-1-59745-543-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shulman RG, Hyder F, Rothman DL. Baseline brain energy supports the state of consciousness. Proc Natl Acad Sci USA. 2009;106:11096–11101. doi: 10.1073/pnas.0903941106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkire MT. Loss of effective connectivity during general anesthesia. Int Anesthesiol Clin. 2008;46:55–73. doi: 10.1097/AIA.0b013e3181755dc6. [DOI] [PubMed] [Google Scholar]
- 21.Hyder F, Rothman DL. Neuronal correlate of BOLD signal fluctuations at rest: err on the side of the baseline. Proc Natl Acad Sci USA. 2010;107:10773–10774. doi: 10.1073/pnas.1005135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northoff G, Duncan NW, Hayes DJ. The brain and its resting state activity—experimental and methodological implications. Prog Neurobiol. 2010;92:593–600. doi: 10.1016/j.pneurobio.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd GM. Neurobiology. New York: Oxford Univ Press; 1994. [Google Scholar]