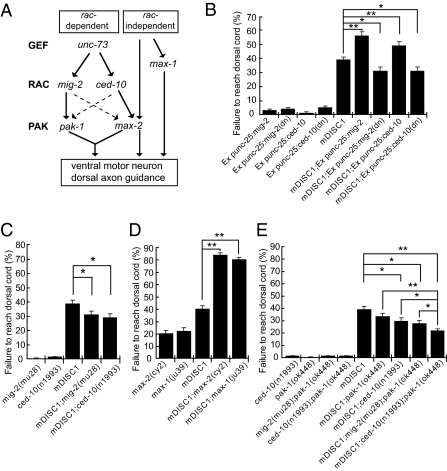
Fig. 3.
mDISC1 in C. elegans motor neurons interacts genetically with RAC-PAK signaling pathways to regulate axon guidance. (A) Schematic diagram summarizing the known RAC-dependent and RAC-independent signaling pathways in developing C. elegans motor neurons (13). The RAC-dependent pathway involves unc-73 (a GEF gene), two functional redundant rac genes, ced-10 and mig-2, and two downstream pak genes, pak-1 and max-2. The RAC-independent pathway is simplified by showing a completely RAC-independent gene, max-1. Note that the max-2 gene also has a RAC-independent function. Solid arrows indicate direct activation, and dashed arrows indicate weaker activation. (B) The severity of axon guidance defects in mDISC1 transgenic animals is significantly suppressed by ced-10(dn) or mig-2(dn), whereas expression of wild-type ced-10 or mig-2 enhances the axon guidance defects of mDISC1 transgenic animals. (C) Single mutants of ced-10 or mig-2 suppress the axon guidance defects of mDISC1 transgenic animals. (D) Axon guidance defects in mDISC1 transgenic animals are greatly enhanced by loss of genes that can transduce signals independent of the RAC signaling pathway. (E) Double mutants of pak-1;ced-10 suppress the defects more significantly than either pak-1 or ced-10 single mutants. They also suppress the defects more significantly than pak-1;mig-2 double mutants, consistent with the preferential uses of the rac-pak genes in the motor axons. In all diagrams, bars represent the SE (n = 32–62). *P < 0.05, **P < 0.001 (Student's t test).