Figure 1.
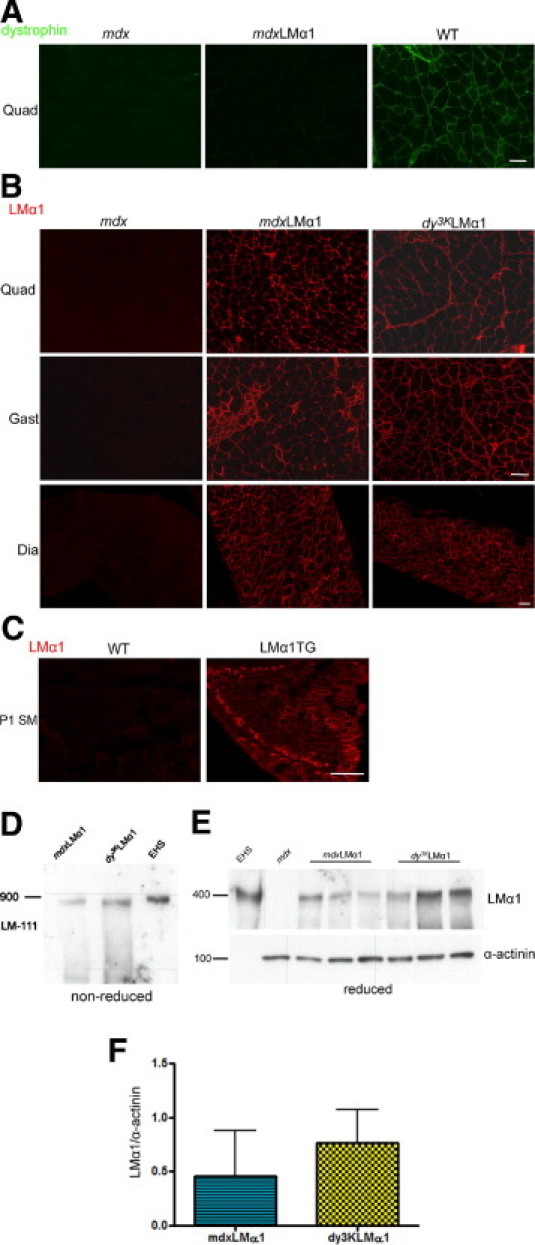
Laminin-111 expression in mdxLMα1 mice. A: Dystrophin immunostaining in mdx, mdxLMα1, and wild-type (WT) skeletal muscle confirms dystrophin absence from mdxLMα1 muscle. B: Laminin α1 chain immunostaining demonstrates uniform expression of laminin α1 subunit in basement membranes of mdxLMα1 skeletal muscle. It is expressed in a similar manner as in laminin α2 chain-deficient mice overexpressing laminin α1 chain (dy3KLMα1). As expected, it is absent from mdx muscle. Quadriceps (Quad), gastrocnemius (Gast), and diaphragm (Dia) muscles are shown. C: Laminin α1 chain is not expressed in wild-type muscle of newborn mice at postnatal day 1 (P1), but is present in muscle from littermates overexpressing transgenic laminin α1 chain. Scale bars = 50 μm (A–C, all images). D: Immunoblotting of skeletal muscle tissue extracts from mdxLMα1 and dy3KLMα1 mice and EHS laminin extract with a rabbit polyclonal antibody against laminin α1 LG3 domain under nonreducing conditions. The laminin-111 (LM-111) heterotrimer is present in mdxLMα1 muscle (900-kDa band). E: Immunoblotting with the same antibody against laminin α1 chain and α-actinin under reducing conditions. A 400-kDa band corresponding to laminin α1 chain is absent from mdx skeletal muscle extract, but is present in mdxLMα1 (n = 3) and dy3KLMα1 (n = 3) muscles. EHS laminin was used as a positive control. F: Quantification of laminin α1 chain signals revealed no significant difference in expression between mdxLMα1 and dy3KLMα1 muscles (P = 0.4000). Laminin α1 chain expression was normalized to α-actinin expression. Mann-Whitney U-test was used for statistical analysis, with significance set at P < 0.05. Results are reported as means ± SD.
