Abstract
Background
Obstructive sleep apnea (OSA) is a very common disorder among adults: the prevalence of mild OSA is 20%, and that of moderate or severe OSA is 6% to 7%. Simple snoring is even more common. Conservative treatments such as nocturnal ventilation therapy and oral appliances are successful as long as the patient actually uses them, but they do not eliminate the underlying obstruction of the upper airway.
Method
The relevant literature up to 2008 on the surgical treatment of OSA was selectively reviewed.
Results
Five types of surgical treatment for OSA are available, each for its own indications: optimization of the nasal airway to support nasal ventilation therapy, (adeno-)tonsillectomy as first-line treatment for OSA in children, minimally invasive surgery for simple snoring and mild OSA, invasive surgery as first- and second-line treatment for mild OSA, and invasive multilevel surgery as second-line treatment of moderate to severe OSA that remains refractory to ventilation therapy.
Conclusion
Surgical treatment for OSA is appropriate for specific indications as a complement to the established conservative treatment methods.
Pathological changes to the upper airway can result in narrowing and so-called obstructive sleep-related breathing disorders. In addition to central causes the international classification of sleep disorders (e1) distinguishes between simple snoring and obstructive sleep apnea (OSA). OSA itself is subcategorized into an adult and pediatric form.
By definition, simple snoring refers to inspiratory breathing sounds that are neither accompanied by insomnia nor hypersomnia nor by a raised cardiovascular risk. Except for possible social problems as a result of the noise produced by breathing while sleeping, simple snoring has no consequences for those affected. The prevalence is age dependent and is reported to be 60% in men over age 60 and 50% in postmenopausal women (1, e2). Intense snoring can be a sign of OSA (2, e3), and in people with intense and arrhythmic snoring, a differential diagnostic evaluation should be conducted by a sleep physician.
The transition to OSA is fluid. OSA is characterized by repeated obstructions in the upper airway during sleep with resultant apneas and hypopneas with or without arousals. Impaired sleep quality reduces the affected person’s quality of life. The main symptoms are sleepiness during the day, arrhythmic snoring, and reduced intellectual performance (e4). Compared with healthy subjects, patients with sleep apnea have an increased risk for morbidity and mortality (3– 6, e5– e9) (Box).
Box. Possible sequelae of obstructive sleep apnea.
Cardiovascular disease
Diabetes
Obesity
Metabolic syndrome
Risk of accidents when vehicles are handled
The main tool for quantifying the severity of OSA is the apnea-hypopnea index (AHI) (Table 1). With regard to current diagnostic procedures we refer our readers to the recent literature (7, e10, e11). In children, as few as two breathing pauses per hour of sleep (AHI >2) is seen as problematic (e12).
Table 1. Severity levels of obstructive sleep apnea according to the apnea-hypopnea index as per ICSD (e1).
| Finding | AHI index |
| Primary snoring | AHI <5 |
| Mild OSA | 5 ≤ AHI <20 |
| Moderate OSA | 20 ≤ AHI <40 |
| Severe OSA | 40 <ahi |
AHI, apnea-hypopnea index; ICSD, International Classification of Sleep Disorders
Selective literature search
We searched PubMed up to 31 December 2008 using the search terms “sleep apnea” and “snoring”, each in combination with “surgery”. We restricted our search to articles written in the German and English languages. Cross references in the respective reference lists were also included. This review article is based on the data collection of the current S2e guidelines “Therapy of obstructive sleep apnea in adults” of the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (8). This guideline in turn refers to the S2 guideline “Non-restorative sleep” of the German Society of Sleep Research and Sleep Medicine (DGSM) (e13) by providing additional comments on the surgical treatment options from the perspective of otorhinolaryngological surgery.
Current treatment for obstructive sleep disorders may be conservative, surgical, or device-based. This article aims to assess the importance of surgical therapy; for details on conservative and device-based treatment options we refer our readers to the pertinent literature.
Conservative treatment
Conservative therapeutic measures include weight reduction (4, e14), optimizing sleep hygiene, adopting the correct position for sleeping (e15, e16), and different drug based approaches (e17). None of these methods tackles the pathological changes that lead to a narrowed upper airway, and for this reason we again refer readers to the relevant specialist literature. Weight loss as well as avoidance of the supine position (lying on one’s back) may be able to support treatment of the obstruction in certain findings.
Device-based therapy
Nasal ventilation, oral and nasal appliances, and electrostimulation are available device-based interventions. A training effect has been found only for muscle stimulation. Because of its effectiveness (e18– e20) and the high quality of the evidence based data, ventilation therapy is the standard treatment for OSA. Long term acceptance, however, depends on aftercare, side effects, and other factors and is often impaired (e21– e24). Among oral appliances, mandibular advancement splints are recommended for moderate to severe OSA (e25– e28). No proof of effectiveness exists thus far for nasal appliances and electrostimulation in OSA (8). For primary snoring, individual case series have shown effectiveness (e29, e30).
Surgical therapy
The current German guideline (8) for obstructive sleep apnea in adults sets out 4 indications for surgery that we will discuss in the following section. An additional indication relates to pediatric OSA.
Distinction has to be made between invasive and minimally invasive surgical approaches. A surgical method is regarded as minimally invasive if the intervention can be delivered under local anesthesia and as an outpatient procedure, and if perioperative and postoperative morbidity is low and complications rare.
Furthermore, distinction is made between primary, secondary, and adjuvant indications for surgery. Surgery as first-line treatment is regarded as equal to ventilation therapy; a second-line treatment should be considered only if device-based therapies have remained unsuccessful; and adjuvant surgery supports a different primary therapeutic option without being sufficiently successful for the obstructive sleep-related breathing disorder all by itself.
The successful surgery for OSA is defined as a reduction of the AHI by at least 50% and to a value below 20 (9); this is in contradiction to the therapeutic criteria for nocturnal ventilation treatment. With regard to these criteria, it has often been critically remarked that reducing the original AHI from 40 to 15, for example, should not be rated as successful treatment. By contrast, device-based therapy works only when appliances are being used, whereas a surgical result yields success every night even without appliances. If in the concrete scenario a reduction of the AHI from 40 to 5 is postulated as a result of continuous positive airway pressure (CPAP), then the corresponding reduction in the AHI as a result of CPAP would be 78%, and 62.5% after surgery. If the CPAP equipment is used only during 80% of nocturnal sleep, however, the results are identical (78 x 0.8 = 62.4). Many definitions of success use far less than 80% use of CPAP for their definition of CPAP compliance. For this reason, the simple comparison of AHI values does not do justice to the differentiated evaluation of the different treatment modalities. Rather, it is desirable that subjective parameters—for example, daytime symptoms and quality of life—and the assessment of the cardiovascular risk be integrated into the evaluation.
Basically, surgical intervention is indicated only if the patient’s general condition allows surgery and a rectifiable pathoanatomical finding has been carefully diagnostically evaluated before the operation.
Improving nasal air flow
An exclusive operation on the nose by itself cannot lower the AHI significantly (8). However, surgery can improve sleep quality, the restorative function of sleep, and CPAP compliance as well as reduce therapeutic urgency (e31– e36) (Table 2). For this reason, surgical correction of relevant impairments to nasal breathing is indicated primarily in case of subjective problems and as adjuvant treatment in problematic CPAP compliance.
Table 2. Effect of nasal surgery on the effective CPAP in patients with OSA.
| Author (reference) | No | CPAP pre (cm H2O) | CPAP post (cm H2O) | p-value |
| Mayer-Brix et al. 1989 (e31) | 3 | 9.7 | 6 | None |
| Friedman et al. 2000 (e32) | 6 | 9.3 | 6.7 | <0.05 |
| Dorn et al. 2001 (e33) | 5 | 11.8 | 8.6 | <0.05 |
| Masdon et al. 2004 (e34) | 35 | 9.7 | 8.9 | n. s. |
| Nakata et al. 2005 (e35) | 5 | 16.8 | 12 | <0.05 |
| Zonato et al. 2006 (e36) | 17 | 12.4 | 10.2 | <0.001 |
| Total | 71 | 11 | 9.1 |
CPAP, continuous positive airway pressure ; pre, preoperatively; post, postoperatively; OSA, obstructive sleep apnea; n.s., non-significant
Adenotonsillectomy in pediatric OSA
In pediatric OSA, adenotonsillar hyperplasia is the main culprit. The effectiveness of adenotonsillectomy (ATE) has been convincingly shown in recent review articles (10, e37). Our own literature analysis of 24 publications included 653 children and found a surgical success rate for ATE in OSA of 83.6% (e38). Furthermore, oxidative stress was reduced (e39) as was total cholesterol (e40), and cognitive performance improved (e41, e42). ATE is therefore indicated as the primary therapeutic option in pediatric OSA. Risk factors for a lack of success include overweight and a raised AHI at baseline (e43). Follow-up monitoring is always advised in such cases.
Because of the notably reduced postoperative pain and rate of postoperative hemorrhage, tonsillotomy is increasingly used in pediatric OSA—with the volume reduction as large as possible. Initial results have shown comparable efficacy for both procedures (11, 12).
Minimally invasive surgery in primary snoring and mild OSA
Interstitial radiofrequency therapy (IRFT) with high-frequency current and soft palate implants are minimally invasive interventions. Because of the substantial postoperative wound pain, resections to the soft palate are somewhat loosely termed minimally invasive.
IRFT is used for the soft palate, palatine tonsils, and the base of the tongue. In the muscles of the soft palate and tongue, tissue stiffening is achieved via postoperative scarring, and in the lymphatic tissue of the tonsils, the volume effect is up to 75% (13). Sparing the mucosa leads to less postoperative pain and fewer complications. Ulcers, hemorrhages, and prolonged odynophagia are the most important complications and occur in less than 1% of cases (14, e44). The soft palate can be stiffened by implanting three cylindrical pillar implants made from polyethylene terephthalate (15).
Table 3 shows the results for OSA. Several minimally invasive procedures can be combined. For primary snoring, numerous articles have shown the subjective effectiveness of minimally invasive treatment of the soft palate, with success rates of more than 70% in some cases, but the same is not true for the base of the tongue.
Table 3. Minimally invasive surgery in OSA.
| Procedure | No of studies | No (patients) | ESS pre | ESS post | AHI pre | AHI post | Success (%) | EBM |
| IRFT soft palate | 3 [e56–e58] | 61 | 20.2 | 12.8 | 47.5 | B | ||
| Implant soft palate | 8 [e59–e66] | 248 | 10.6 | 7.4 | 20.3 | 17.3 | 30.4 | B |
| Resection | 18 [e58, e67–e83] | 576 | 10.2 | 7.5 | 25.6 | 19.4 | 42.4 | B |
| IRFT base of tongue | 6 [e84–e84] | 133 | 10.3 | 6.1 | 37.3 | 25.8 | 33.2 | B |
| Combined therapies | 5 [e90–e94] | 203 | 10.2 | 7.3 | 24.7 | 16 | 45.7 | B |
ESS, Epworth sleepiness scale; pre, preoperatively; post, postoperatively; AHI, Apnea-hypopnea index; EBM, level of recommendation according to evidence based medicine; IRFT, interstitial radiofrequency therapy
For this reason, a primary indication in simple snoring exists only for the soft palate. In OSA, primary use on the soft palate and the base of the tongue is indicated only for mild forms. Data on the treatment of the palatine tonsils are lacking.
Invasive surgery in OSA
The most widely established surgical procedure is uvulopalatopharyngoplasty (UPPP) (e45, e46). The principle is a widening of the oropharyngeal valve in the transverse as well as a sagittal direction. Selecting suitable patients is crucial for therapeutic success. The specialist literature provides more detail on the topodiagnostic evaluation of the location of the collapse (16, e47).
A recent meta-analysis of 269 cases of UPPP found an objective treatment success as defined by Sher (9) of 30% without and 59% with simultaneous tonsillectomy (e48). Positive predictors for therapeutic success are hyperplastic tonsils, substantial excess mucosa of the soft palate, a long uvula, longitudinal folds of the mucosa covering the back wall of the pharynx, and an observed obstruction of the soft palate on sleep endoscopy. The long term success rate falls from 60.5% after 3 to 12 months to 47.6% after 3 to 7 years (8).
Subjectively, the success after UPPP is comparable to that of CPAP therapy (e49). With regard to mortality, patients with sleep apnea have been found to have no significant difference in mortality up to 9 years as compared to a matched control cohort (17). The long term survival rate for CPAP and after UPPP is identical, as far is known from research so far (18). UPPP is the only surgical treatment for OSA for which a reduction in the risk of incidents (19, e50) and normalization of raised specific values for serum C-reactive protein (20) have been shown. UPPP with tonsillectomy seems indicated for the treatment of mild to moderate OSA if the pathoanatomical findings justify the method. Whether modifications of UPPP are likely to yield better results is currently not known.
In contrast to the soft palate, no established standard technique exists for surgical treatment of retrolingual and hypopharyngeal obstruction. Most procedures are used in combination with other interventions to the upper airway. Table 4 shows the available data for the isolated use of the respective surgical techniques. With regard to surgical technique, indications, and risks, we refer readers to the literature (21, 22).
Table 4. Invasive surgery to the base of the tongue/hypopharynx without additional interventions in obstructive sleep apnea.
| No of studies | No (patients) | AHI pre | AHI post | Success (%) | EBM | |
| Hyoid suspension | 3 [e95–e97] | 60 | 36 | 21.1 | 49.5 | C |
| Partial resection of the tongue | 5 [e98–e103] | 96 | 49.5 | 25.4 | 54.4 | B |
| Tongue suspension | 3 [e104–e106] | 37 | 33.6 | 19.8 | 28.6 | C |
| Genioglossus advancement* | None | D |
AHI, apnea-hypopnea index; pre, preoperatively; post, postoperatively; EBM, level of recommendation according to evidence based medicine;
*osteosynthetic advancement of the genioglossus muscle
Maxillomandibular advancement (MMA) osteotomy simultaneously widens the nasopharynx, oropharynx, and hypopharynx by advancing the soft palate and tongue and tightening the lateral pharyngeal walls. Although MMA is regarded as standard treatment it remains technically demanding and requires full anesthesia and inpatient treatment (23). After tracheotomy, MMA is the most successful surgical approach for treating OSA (8). Compared with CPAP, similar reductions of the AHI are achieved, as are a similar optimization of sleep architecture (24). Treatment effects usually last for a minimum of 50 months (25, e51).
MMA is indicated for patients with suitable anatomical conditions. Morbidity, the complication rate, and cosmetic sequelae should be borne in mind when defining the indication.
Laryngeal or tracheal obstructions will trigger OSA only in rare cases; treatment should be administered according to the underlying impairment (22).
Polysomnographic data from 4 observational studies with a total of 159 patients have shown an average success rate for tracheotomy of 96.2% (8). In spite of this, tracheotomy is the approach of last resort in selected cases because of the substantial impairment to patients’ quality of life.
Multilevel surgery in OSA
Nowadays, invasive surgical procedures are rarely undertaken in isolation but usually in combination. In the following section we will refer to multilevel surgery (MLS) if at least one intervention to the base of the tongue/hypopharynx is combined with at least one intervention to the soft palate/tonsil. Therapeutic schemes for moderate to severe OSA at the level of the soft palate always include tonsillectomy combined with UPPP or one of its numerous modifications. For the treatment of hypopharyngeal narrowing, different methods are recommended.
Currently, 11 controlled studies and 32 case series exist, including a total of 1640 patients (8). The average AHI preoperatively was 43.9 and, postoperatively, 20.3. The success rate according to the Sher criteria was 53.8%. The data situation is sufficient to be able to assume a general effectiveness of MLS in severe OSA (recommendation level B). However, it is not yet clear or foreseeable which combination of procedures will be superior. MLS yielded poorer results than nasal CPAP therapy; an indication therefore only exists as secondary therapy for patients who are not, or no longer, open to ventilation therapy.
Outlook
Sleep medicine is a cross sectional discipline to which many specialties contribute; this naturally results in the assessment of any therapeutic measure from different perspectives. Nonetheless, consensus nowadays exist regarding the use of rhinosurgery as an adjuvant measure for ventilation therapy and the benefit of adenotonsillectomy as the primary therapeutic approach in pediatric OSA. Consensus also exists with regard to the fact that patients with severe OSA and/or pathological overweight and/or substantial comorbidities should be given ventilation treatment as the primary measure. Multilevel surgery can be a secondary therapeutic option in this setting. Multimodal therapeutic approaches, such as surgical intervention and wearing an oral appliance, may also be successful in case CPAP proves a therapeutic failure (e52).
Minimally invasive therapies are accepted in primary snoring because of their low rate of complications. The success rates of minimally invasive therapy in snoring seemed to be comparable to the evidence on the efficacy of oral appliances. The advantage of an oral device lies in the fact that the treatment can be interrupted at any time and undesired side effects may reverse. Surgery, on the other hand, has the advantage that the affected person does not require a permanent contraption.
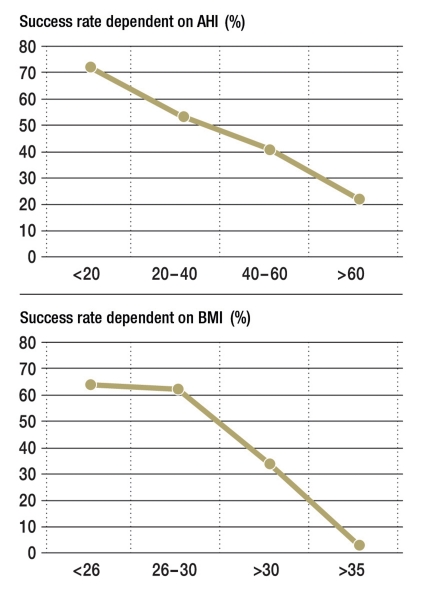
Which intervention should be the primary one for OSA is the subject of controversial discussion. The high success rate and better-quality studies for CPAP treatment militate against using surgery. The potential and sometimes irreversible complications and the difficulty in selecting the adequate surgical approach, or multilevel surgery plan, are further arguments against surgery as the primary treatment modality. However, patients’ acceptance of CPAP therapy in mild OSA without clinical symptoms is particularly low. At the same time, low baseline values for AHI and BMI (body mass index) (Figure) and a lower rate of daytime symptoms are positive predictors for successful surgery. The milder the OSA the less comprehensively and invasively surgery needs to be. This means that perioperative and postoperative morbidity and complications are also a function of baseline AHI values. The subjective success of surgery regarding daytime symptoms, the partner’s acceptance, and the snoring itself are no worse for the surgical approaches than for device-based therapy. This, combined with the fact that after successful surgery patients are not dependent on a technical appliance, explains the popularity of surgical therapy in patients.
Figure.
Objective surgical success in sleep surgical interventions for the treatment of obstructive sleep apnea depending on the apnea-hypopnea index (AHI) and body mass index (BMI). Success rate as defined by Sher (9)—that is, reduction of AHI by at least 50% and reduction of AHI to below 20), N = 263 patients; with permission from (22)
Against this background, a general rejection of primary surgery for OSA, such as was postulated in the latest Cochrane review of 2005 and several evidence based analyses (e53– e55) seems not exactly differentiated. Primary use of surgery to eliminate obstruction in OSA may be justified in patients whose obstruction is easy to remove and if the OSA is mild. On the basis of today’s state of research, however, it is difficult to establish precise threshold values for AHI, BMI, or comorbidity that define whether surgery should be the primary or secondary therapeutic approach.
Key Messages.
The prevalence of mild obstructive sleep apnea (OSA) in adults is 20%; with 6–7% experiencing the severe form.
Device-based therapies, such as nocturnal ventilation and oral devices are successful in patients whose compliance is good.
In children with OSA, adenotonsillectomy has a success rate of 83.6%.
In adults, several operative procedures and a primary treatment are available to treat obstruction in primary snoring.
In severe OSA, surgery should be undertaken only to optimize ventilation treatment or as a secondary option if ventilation treatment fails or patients are non-compliant.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
Professor Hörmann declares that no conflict of interest exists.
Professor Verse has received honoraria for speaking, advisory fees, travel expenses, or research funds from: Celon AG, Aspire Medical, Apnoen Medical, Inspire Medical, ResMed GmbH, and MPV Truma GmbH.
References
- 1.Young T, Skatrud J, Peppard P. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 2.Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest. 2006;130:149–156. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest. 2007;132:1858–1862. doi: 10.1378/chest.07-1170. [DOI] [PubMed] [Google Scholar]
- 4.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 5.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–408. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George F, George P. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–956. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 7.Maurer JT, Stuck BA. Update Diagnostik beim obstruktiven Schlafapnoesyndrom. HNO. 2008;56:1089–1097. doi: 10.1007/s00106-008-1758-2. [DOI] [PubMed] [Google Scholar]
- 8.Verse T, Bodlaj R, de la Chaux R, et al. ArGe Schlafmedizin der Deutschen Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie. Leitlinie: Therapie der obstruktiven Schlafapnoe des Erwachsenen. HNO. 2009;57:1136–1156. doi: 10.1007/s00106-009-2013-1. [DOI] [PubMed] [Google Scholar]
- 9.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 10.Brietzke SE, Gallagher D. The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/Hypopnea syndrome: a meta-analysis. Otolaryngol Head Neck Surg. 2006;134:979–984. doi: 10.1016/j.otohns.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Colen TY, Seidman C, Weedon J, Goldstein NA. Effect of intracapsular tonsillectomy on quality of life for children with obstructive sleep-disordered breathing. Arch Otolaryngol Head Neck Surg. 2008;134:124–127. doi: 10.1001/archoto.2007.8. [DOI] [PubMed] [Google Scholar]
- 12.de la Chaux R, Klemens C, Patscheider M, Reichel O, Dreher A. Tonsillotomy in the treatment of obstructive sleep apnea syndrome in children: polysomnographic results. Int J Pediatr Otorhinolaryngol. 2008;72:1411–1147. doi: 10.1016/j.ijporl.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Nelson LM. Temperature-controlled radiofrequency tonsil reduction in children. Arch Otolaryngol Head Neck Surg. 2003;129:533–537. doi: 10.1001/archotol.129.5.533. [DOI] [PubMed] [Google Scholar]
- 14.Toh ST, Hsu PP, Ng YH, Teo TW, Tan KL, Lu KS. Incidence of complications after temperature-controlled radiofrequency treatment for sleep-disordered breathing: a Singapore sleep centre experience. J Laryngol Otol. 2008;122:490–494. doi: 10.1017/S0022215107009528. [DOI] [PubMed] [Google Scholar]
- 15.Maurer JT, Verse T, Stuck BA, Hörmann K, Hein G. Palatal implants for primary snoring: short-term results of a new minimally invasive surgical technique. Otolaryngol Head Neck Surg. 2005;132:125–131. doi: 10.1016/j.otohns.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Maurer JT, Stuck BA. Update: Diagnostik der oberen Atemwege bei Schlafapnoe-Syndrom. HNO. 2008;56:1089–1097. doi: 10.1007/s00106-008-1758-2. [DOI] [PubMed] [Google Scholar]
- 17.Lysdahl M, Haraldson PO. Long-term survival after uvulopalatopharyngoplasty in nonobese heavy snorers: a 5- to 9-year follow-up of 400 consecutive patients. Arch Otolaryngol Head Neck Surg. 2000;126:1136–1140. doi: 10.1001/archotol.126.9.1136. [DOI] [PubMed] [Google Scholar]
- 18.Keenan SP, Burt H, Ryan CF, Fleetham JA. Long-term survival of patients with obstructive sleep apnea treated by uvulopalatopharyngoplasty or nasal CPAP. Chest. 1994;105:155–159. doi: 10.1378/chest.105.1.155. [DOI] [PubMed] [Google Scholar]
- 19.Haraldsson PO, Carenfelt C, Lysdahl M, Tingvall C. Does uvulopalatopharyngoplasty inhibit automobile accidents? Laryngoscope. 1995;105:657–661. doi: 10.1288/00005537-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita H, Shibano A, Sakoda T, et al. Uvulopalatopharyngoplasty decreases levels of C-reactive protein in patients with obstructive sleep apnea syndrome. Am Heart J. 2006;152 692:e1–e5. doi: 10.1016/j.ahj.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Kezirian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Arch Otolaryngol Head Neck Surg. 2006;132:206–213. doi: 10.1001/archotol.132.2.206. [DOI] [PubMed] [Google Scholar]
- 22.Verse T. Update: Operative Möglichkeiten zur Behandlung der obstruktiven Schlafapnoe. HNO. 2008;56:1098–1104. doi: 10.1007/s00106-008-1813-z. [DOI] [PubMed] [Google Scholar]
- 23.Prinsell JR. Maxillomandibular advancement (MMA) in a site-specific treatment approach for obstructive sleep apnea: a surgical algorithm. Sleep Breath. 2000;4:147–154. doi: 10.1007/s11325-000-0147-1. [DOI] [PubMed] [Google Scholar]
- 24.Conradt R, Hochban W, Heitmann J, et al. Sleep fragmentation and daytime vigilance in patients with OSA treated by surgical maxillomandibular advancement compared to CPAP therapy. J Sleep Res. 1998;7:217–223. doi: 10.1046/j.1365-2869.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 25.Li KK, Powell NB, Riley RW, Troell RJ, Guilleminault C. Long-term results of maxillomandibular advancement surgery. Sleep Breath. 2000;4:137–139. doi: 10.1007/s11325-000-0137-3. [DOI] [PubMed] [Google Scholar]
- e1.American Academy of Sleep Medicine. 2nd edition. Westchester/IL, USA: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders. Diagnostic and coding manual. [Google Scholar]
- e2.Young T, Palat M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- e3.Michaelson PG, Allan P, Chaney J, Mair EA. Validations of a portable home sleep study with twelve-lead polysomnography: comparisons and insights into a variable gold standard. Ann Otol Rhinol Laryngol. 2006;115:802–809. doi: 10.1177/000348940611501102. [DOI] [PubMed] [Google Scholar]
- e4.Guilleminault C, Dement WC, editors. New York: Alan R. Liss; 1978. Sleep apnea syndrome. [Google Scholar]
- e5.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnoe index in obstructive sleep apnea: Experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- e6.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Otto ME, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298–1302. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- e8.Vorona RD, Ware JC. Sleep disordered breathing and driving risk. Curr Opin Pulm Med. 2002;8:506–510. doi: 10.1097/00063198-200211000-00004. [DOI] [PubMed] [Google Scholar]
- e9.Ellen RL, Marshall SC, Palayew M, et al. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193–200. [PubMed] [Google Scholar]
- e10.Verse T, Pirsig W. Aktuelles zur Diagnostik schlafbezogener Atmungsstörungen. HNO. 2001;49:6–11. doi: 10.1007/s001060050701. [DOI] [PubMed] [Google Scholar]
- e11.Fischer Y, Neagos A, Pirsig W. Schlafbezogene Atmungsstörungen. Schlafanamnesebögen und klinische Befunderhebung im Rahmen der Stufendiagnostik. HNO. 2005;53:995–1008. doi: 10.1007/s00106-005-1314-2. [DOI] [PubMed] [Google Scholar]
- e12.Halbower AC, Ishman SL, McGinley BM. Childhood obstructive sleep-disordered breathing. A clinical update and discussion of technological innovations and challenges. Chest. 2007;132:2030–2041. doi: 10.1378/chest.06-2827. [DOI] [PubMed] [Google Scholar]
- e13.Fischer J, Mayer G, Peter JH, Riemann D, Sitter H. Leitlinie “S2” der Deutschen Gesellschaft für Schlafforschung und Schlafmedizin (DGSM) Somnologie. 2001;5(Suppl 3):1–258. [Google Scholar]
- e14.Barvaux VA, Aubert G, Rodenstein DO. Weight loss as a treatment for obstructive sleep apnoea. Sleep Med Rev. 2000;4:435–452. doi: 10.1053/smrv.2000.0114. [DOI] [PubMed] [Google Scholar]
- e15.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115:771–781. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- e16.Wenzel S, Smith E, Leiacker R, Fischer Y. Effektivität und Langzeit-Compliance der Therapie mit Rückenlage-Verhinderungsweste bei obstruktiver Schlafapnoe. Laryngorhinootologie. 2007;86:579–583. doi: 10.1055/s-2007-966179. [DOI] [PubMed] [Google Scholar]
- e17.Smith I, Lasserson T, Wright J. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(2) doi: 10.1002/14651858.CD003002.pub2. CD003002. [DOI] [PubMed] [Google Scholar]
- e18.Jenkinson C, Davies RJO, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- e19.Ballester E, Badia JR, Hernandez L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- e20.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD001106.pub3. CD 001106. [DOI] [PubMed] [Google Scholar]
- e21.McArdle N, Dervereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea / hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- e22.Janson C, Nöges E, Svedberg-Brandt S, Lindberg E. What characterizes patients who are unable to tolerate continuous positive airway pressure (CPAP) treatment? Resp Med. 2000;94:145–149. doi: 10.1053/rmed.1999.0703. [DOI] [PubMed] [Google Scholar]
- e23.Lin HS, Prasad AS, Pan CJ, Rowley JA. Factors associated with noncompliance to treatment with positive airway pressure. Arch Otolaryngol Head Neck Surg. 2007;133:69–72. doi: 10.1001/archotol.133.1.69. [DOI] [PubMed] [Google Scholar]
- e24.Souter MA, Stevenson S, Sparks B, Drennan C. Upper airway surgery benefits patients with obstructive sleep apnoea who cannot tolerate nasal continuous positive airway pressure. J Laryngol Otol. 2004;118:270–274. doi: 10.1258/002221504323012003. [DOI] [PubMed] [Google Scholar]
- e25.Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693–699. doi: 10.1378/chest.06-2038. [DOI] [PubMed] [Google Scholar]
- e26.Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109:1269–1275. doi: 10.1378/chest.109.5.1269. [DOI] [PubMed] [Google Scholar]
- e27.Pantin CC, Hillman DR, Tennant M. Dental side effects of an oral device to treat snoring and obstructive sleep apnea. Sleep. 1999;22:237–240. doi: 10.1093/sleep/22.2.237. [DOI] [PubMed] [Google Scholar]
- e28.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev 2004, 18 CD004435. Update in Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD004435.pub3. CD004435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e29.Randerath WJ, Galetke W, Domanski U, Weitkunat R, Rühle KH. Tongue-muscle training by intraoral electrical neurostimulation in patients with obstructive sleep apnea. Sleep. 2004;27:254–259. doi: 10.1093/sleep/27.2.254. [DOI] [PubMed] [Google Scholar]
- e30.Verse T, Schwalb J, Hörmann K, Stuck BA, Maurer JT. Transkutane, submentale Elektrostimulationstherapie zur Behandlung der obstruktiven Schlafapnoe. HNO. 2003;51:966–970. doi: 10.1007/s00106-003-0842-x. [DOI] [PubMed] [Google Scholar]
- e31.Mayer-Brix J, Müller-Marschhausen U, Becker H, Peter JH. Wie häufig sind pathologische HNO-Befunde bei Patienten mit obstruktivem Schlaf-Apnoe-Syndrom? HNO. 1989;37:511–516. [PubMed] [Google Scholar]
- e32.Friedman M, Tanyeri H, Lim JW, Landsberg R, Vaidyanathan K, Caldarelli D. Effect of nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;122:71–74. doi: 10.1016/S0194-5998(00)70147-1. [DOI] [PubMed] [Google Scholar]
- e33.Dorn M, Pirsig W, Verse T. Postoperatives Management nach rhinochirurgischen Eingriffen bei Patienten mit schwerer obstruktiver Schlafapnoe: eine Pilotstudie. HNO. 2001;49:642–645. doi: 10.1007/s001060170062. [DOI] [PubMed] [Google Scholar]
- e34.Masdon JL, Magnuson JS, Youngblood G. The effects of upper airway surgery for obstructive sleep apnea on nasal continuous positive airway pressure settings. Laryngoscope. 2004;114:205–207. doi: 10.1097/00005537-200402000-00005. [DOI] [PubMed] [Google Scholar]
- e35.Nakata S, Noda A, Yagi H, et al. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology. 2005;44:296–299. [PubMed] [Google Scholar]
- e36.Zonato AI, Bittencourt LR, Martinho FL, Gregório LC, Tufik S. Upper airway surgery: the effect on nasal continuous positive airway pressure titration on obstructive sleep apnea patients. Eur Arch Otorhinolaryngol. 2006;263:481–486. doi: 10.1007/s00405-005-1018-y. [DOI] [PubMed] [Google Scholar]
- e37.Shine NP, Coates HL, Lannigan FJ. Obstructive sleep apnea, morbid obesity, and adenotonsillar surgery: a review of the literature. Int J Pediatr Otorhinolaryngol. 2005;69:1475–1482. doi: 10.1016/j.ijporl.2005.08.008. [DOI] [PubMed] [Google Scholar]
- e38.Verse T. Update operative Möglichkeiten zur Behandlung der obstruktiven Schlafapnoe. HNO. 2008;56:1098–1104. doi: 10.1007/s00106-008-1813-z. [DOI] [PubMed] [Google Scholar]
- e39.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116:2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- e40.Waters KA, Sitha S, O’Brien LM, et al. Follow-up on metabolic markers in children treated for obstructive sleep apnea. Am J Respir Crit Care Med. 2006;174:455–460. doi: 10.1164/rccm.200401-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e41.Mitchell RB, Kelly J. Behavorial changes in children with mild sleep-disordered breathing or obstructive sleep apnea after adenotonsillectomy. Laryngoscope. 2007;117:1685–1688. doi: 10.1097/MLG.0b013e318093edd7. [DOI] [PubMed] [Google Scholar]
- e42.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117:769–778. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e43.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149:803–808. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- e44.Stuck BA, Starzak K, Verse T, Hörmann K, Maurer JT. Complications of temperature controlled radiofrequency volumetric tissue reduction for sleep disordered breathing. Acta Otolaryngol. 2003;123:532–535. doi: 10.1080/00016480310001385. [DOI] [PubMed] [Google Scholar]
- e45.Ikematsu T. Study of snoring. Therapy [in Japanese] J Jpn Otol Rhinol Laryngol Soc. 1964;64:434–435. [Google Scholar]
- e46.Fujita S, Conway W, Zorick F. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981;89:923–934. doi: 10.1177/019459988108900609. [DOI] [PubMed] [Google Scholar]
- e47.Stuck BA, Maurer JT. Airway evaluation in obstructive sleep apnea. Sleep Med Rev. 2008;12:411–436. doi: 10.1016/j.smrv.2007.08.009. [DOI] [PubMed] [Google Scholar]
- e48.Maurer JT. Update on surgical treatments for sleep apnea. Swiss Med Wkly. 2009;139:624–629. doi: 10.4414/smw.2009.12652. [DOI] [PubMed] [Google Scholar]
- e49.Lojander J, Maasilta P, Partinen M, Brander PE, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110:114–119. doi: 10.1378/chest.110.1.114. [DOI] [PubMed] [Google Scholar]
- e50.Haraldson PO, Carenfelt C, Persson HE, Sachs C, Tornros J. Simulated long-term driving performance before and after uvulopalatopharyngoplasty. J Otorhinolaryngol Relat Spec. 1991;53:106–110. doi: 10.1159/000276198. [DOI] [PubMed] [Google Scholar]
- e51.Riley RW, Powell NB, Guilleminault C. Maxillofacial surgery and nasal CPAP. A comparison of treatment for obstructive sleep apnea syndrome. Chest. 1990;98:1421–1425. doi: 10.1378/chest.98.6.1421. [DOI] [PubMed] [Google Scholar]
- e52.Maurer JT. Kombinierte operative und prothetische Therapie bei schwerer obstruktiver Schlafapnoe - Ein Fallbericht. Laryngorhinootol. 2001;80:278–281. doi: 10.1055/s-2001-13890. [DOI] [PubMed] [Google Scholar]
- e53.Sundaram S, Bridgman SA, Lim J, Lasserson TJ. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4) doi: 10.1002/14651858.CD001004.pub2. CD001004. [DOI] [PubMed] [Google Scholar]
- e54.Elshaug AG, Moss JR, Southcott AM, Hiller JE. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep. 2007;30:461–467. doi: 10.1093/sleep/30.4.461. [DOI] [PubMed] [Google Scholar]
- e55.Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea—a systematic review. Sleep. 2009;32:27–36. [PMC free article] [PubMed] [Google Scholar]
- e56.Brown DJ, Kerr P, Kryger M. Radiofrequency tissue reduction of the soft palate in patients with moderate sleep-disordered breathing. J Otolaryngol. 2001;30:193–198. doi: 10.2310/7070.2001.19696. [DOI] [PubMed] [Google Scholar]
- e57.Blumen MB, Dahan S, Fleury B, Hausser-Hauw C, Chabolle F. Radiofrequency ablation for the treatment of mild to moderate obstructive sleep apnea. Laryngoscope. 2002;112:2086–2092. doi: 10.1097/00005537-200211000-00033. [DOI] [PubMed] [Google Scholar]
- e58.Bassiouny A, El Salamawy A, Abd El-Tawab M, Atef A. Bipolar radiofrequency treatment for snoring with mild to moderate sleep apnea: a comparative study between the radiofrequency assisted uvulopalatoplasty technique and the channeling technique. Eur Arch Otorhinolaryngol. 2007;264:659–667. doi: 10.1007/s00405-007-0244-x. [DOI] [PubMed] [Google Scholar]
- e59.Friedman M, Vidyasagar R, Bliznikas D, Joseph NJ. Patient selection and efficacy of pillar implant technique for treatment of snoring and obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2006;134:187–196. doi: 10.1016/j.otohns.2005.10.032. [DOI] [PubMed] [Google Scholar]
- e60.Friedman M, Schalch P, Joseph NJ. Palatal stiffening after failed uvulopalatopharyngoplasty with the Pillar Implant System. Laryngoscope. 2006;116:1956–1961. doi: 10.1097/01.mlg.0000242119.92179.b6. [DOI] [PubMed] [Google Scholar]
- e61.Walker RP, Levine HL, Hopp ML, Greene D, Pang K. Palatal implants: a new approach for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2006;135:549–554. doi: 10.1016/j.otohns.2006.02.020. [DOI] [PubMed] [Google Scholar]
- e62.Nordgard S, Stene BK, Skjøstad KW. Soft palate implants for the treatment of mild to moderate obstructive sleep apnea. Otolaryngol Head Neck Surg. 2006;134:565–570. doi: 10.1016/j.otohns.2005.11.034. [DOI] [PubMed] [Google Scholar]
- e63.Nordgard S, Hein G, Stene BK, Skjøstad KW, Maurer JT. One-year results: palatal implants for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;136:818–822. doi: 10.1016/j.otohns.2006.11.018. [DOI] [PubMed] [Google Scholar]
- e64.Goessler UR, Hein G, Verse T, Stuck BA, Hörmann K, Maurer JT. Soft palate implants as a minimally invasive treatment for mild to moderate obstructive sleep apnea. Acta Otolaryngol. 2007;127:527–531. doi: 10.1080/00016480600951392. [DOI] [PubMed] [Google Scholar]
- e65.Friedman M, Schalch P, Lin HC, Kakodkar KA, Joseph NJ, Mazloom N. Palatal implants for the treatment of snoring and obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2008;138:209–216. doi: 10.1016/j.otohns.2007.10.026. [DOI] [PubMed] [Google Scholar]
- e66.Steward DL, Huntley TC, Woodson BT, Surdulescu V. Palate implants for obstructive sleep apnea: multi-institution, randomized, placebo-controlled study. Otolaryngol Head Neck Surg. 2008;139:506–510. doi: 10.1016/j.otohns.2008.07.021. [DOI] [PubMed] [Google Scholar]
- e67.Petri N, Suadicani P, Wildschiodtz G, BjornJorgensen J. Predictive value of Mueller maneuver, cephalometry and clinical features for the outcome of uvulopalatopharyngoplasty. Acta Otolaryngol (Stockh) 1994;114:565–571. doi: 10.3109/00016489409126106. [DOI] [PubMed] [Google Scholar]
- e68.Kamami YV. Outpatient treatment of sleep apnea syndrome with CO2 laser: laser-assisted UPPP. J Otolaryngol. 1994;23:395–398. [PubMed] [Google Scholar]
- e69.Terris DJ, Clerk AA, Norbash AM, Troell RJ. Characterization of postoperative edema following laser-assisted uvulopalatoplasty using MRI and polysomnography: implications for the outpatient treatment of obstructive sleep apnea syndrome. Laryngoscope. 1996;106:124–128. doi: 10.1097/00005537-199602000-00002. [DOI] [PubMed] [Google Scholar]
- e70.Lauretano AM, Khosla RK, Richardson G, et al. Efficacy of laser-assisted uvulopalatoplasty. Lasers Surg Med. 1997;21:109–116. doi: 10.1002/(sici)1096-9101(1997)21:2<109::aid-lsm1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- e71.Utley DS, Shin EJ, Clerk AA, Terris DJ. A cost-effective and rational surgical approach to patients with snoring, upper airway resistance syndrome, or obstructive sleep apnea syndrome. Laryngoscope. 1997;107:726–734. doi: 10.1097/00005537-199706000-00005. [DOI] [PubMed] [Google Scholar]
- e72.Mickelson SA, Ahuja A. Short-term objective and long-term subjective results of laser-assisted uvulopalatoplasty for obstructive sleep apnea. Laryngoscope. 1999;109:362–367. doi: 10.1097/00005537-199903000-00004. [DOI] [PubMed] [Google Scholar]
- e73.Pribitkin EA, Schutte SL, Keane WM, et al. Efficacy of laser-assisted uvulopalatoplasty in obstructive sleep apnea. Otolaryngol Head Neck Surg. 1998;119:643–647. doi: 10.1016/S0194-5998(98)70026-9. [DOI] [PubMed] [Google Scholar]
- e74.Walker RP, Grigg-Damberger MM, Gopalsami C. Laser-assisted uvulopalatopharyngoplasty for the treatment of mild, moderate, and severe obstructive sleep apnea. Laryngoscope. 1999;109:79–85. doi: 10.1097/00005537-199901000-00016. [DOI] [PubMed] [Google Scholar]
- e75.Ryan CF, Love LL. Unpredictable results of laser assisted uvulopalatoplasty in the treatment of obstructive sleep apnoea. Thorax. 2000;55:399–404. doi: 10.1136/thorax.55.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e76.Seemann RP, DiToppa JC, Holm MA, Hanson J. Does laser-assisted uvulopalatoplasty work? An objective analysis using pre- and postoperative polysomnographic studies. J Otolaryngol. 2001;30:212–215. doi: 10.2310/7070.2001.19725. [DOI] [PubMed] [Google Scholar]
- e77.Finkelstein Y, Stein G, Ophir D, Berger R, Berger G. Laser-assisted uvulopalatoplasty for the management of obstructive sleep apnea. Myths and facts. Arch Otolaryngol Head Neck Surg. 2002;128:429–434. doi: 10.1001/archotol.128.4.429. [DOI] [PubMed] [Google Scholar]
- e78.Ferguson KA, Heighway H, Ruby RRF. A randomized trial of laser-assisted uvulopalatoplasty in the treatment of mild obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:15–19. doi: 10.1164/rccm.2108050. [DOI] [PubMed] [Google Scholar]
- e79.Berger G, Stein G, Ophir D, Finkelstein Y. Is there a better way to do laser-assisted uvulopalatoplasty? Arch Otolaryngol Head Neck Surg. 2003;129:447–453. doi: 10.1001/archotol.129.4.447. [DOI] [PubMed] [Google Scholar]
- e80.Kern RC, Kutler DI, Reid KJ, Conley DB, Herzon GD, Zee P. Laser-assisted uvulopalatoplasty and tonsillectomy for the management of obstructive sleep apnea syndrome. Laryngoscope. 2003;113:1175–1181. doi: 10.1097/00005537-200307000-00013. [DOI] [PubMed] [Google Scholar]
- e81.Larrosa F, Hernandez L, Morello A, Ballester E, Quinto L, Montserrat JM. Laser-assisted uvulopalatoplasty for snoring: does it meet the expectations? Eur Respir J. 2004;24:66–70. doi: 10.1183/09031936.04.00082903. [DOI] [PubMed] [Google Scholar]
- e82.Macdonald A, Drinnan M, Johnston A, et al. Evaluation of potential predictors of outcome of laser-assisted uvulopalatoplasty for snoring. Otolaryngol Head Neck Surg. 2006;134:197–203. doi: 10.1016/j.otohns.2005.10.016. [DOI] [PubMed] [Google Scholar]
- e83.Atef A, Mosleh M, Hesham M, Fathi A, Hassan M, Fawzy M. Radiofrequency vs laser in the management of mild to moderate obstructive sleep apnoea: does the number of treatment sessions matter? J Laryngol Otol. 2005;119:888–893. doi: 10.1258/002221505774783485. [DOI] [PubMed] [Google Scholar]
- e84.Powell NB, Riley RW, Guilleminault C. Radiofrequency tongue base reduction in sleep-disordered breathing: a pilot study. Otolaryngol Head Neck Surg. 1999;120:656–664. doi: 10.1053/hn.1999.v120.a96956. [DOI] [PubMed] [Google Scholar]
- e85.Woodson BT, Nelson L, Mickelson S, Huntley T, Sher A. A multi-institutional study of radiofrequency volumetric tissue reduction for OSAS. Otolaryngol Head Neck Surg. 2001;125:303–311. doi: 10.1067/mhn.2001.118958. [DOI] [PubMed] [Google Scholar]
- e86.Stuck BA, Maurer JT, Verse T, Hörmann K. Tongue base reduction with temperature-controlled radiofrequency volumetric tissue reduction for treatment of obstructive sleep apnea syndrome. Acta Otolaryngol. 2002;122:531–536. doi: 10.1080/00016480260092354. [DOI] [PubMed] [Google Scholar]
- e87.Li KK, Powell NB, Riley RW, Guilleminault C. Temperature-controlled radiofrequency tongue base reduction for sleep-disordered breathing: Long-term outcomes. Otolaryngol Head Neck Surg. 2002;127:230–234. doi: 10.1067/mhn.2002.126900. [DOI] [PubMed] [Google Scholar]
- e88.Riley RW, Powell NB, Li KK, Weaver EM, Guilleminault C. An adjunctive method of radiofrequency volumetric tissue reduction of the tongue for OSAS. Otolaryngol Head Neck Surg. 2003;129:37–42. doi: 10.1016/S0194-59980300482-0. [DOI] [PubMed] [Google Scholar]
- e89.den Herder C, Kox D, van Tinteren H, de Vries N. Bipolar radiofrequency induced thermotherapy of the tongue base: Its complications, acceptance and effectiveness under local anesthesia. Eur Arch Otorhinolaryngol. 2006;263:1031–1140. doi: 10.1007/s00405-006-0115-x. [DOI] [PubMed] [Google Scholar]
- e90.Fischer Y, Khan M, Mann WJ. Multilevel temperature-controlled radiofrequency therapy of soft palate, base of tongue, and tonsils in adults with obstructive sleep apnea. Laryngoscope. 2003;113:1786–1791. doi: 10.1097/00005537-200310000-00024. [DOI] [PubMed] [Google Scholar]
- e91.Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2003;128:848–861. doi: 10.1016/S0194-59980300461-3. [DOI] [PubMed] [Google Scholar]
- e92.Stuck BA, Starzak K, Hein G, Verse T, Hörmann K, Maurer JT. Combined radiofrequency surgery of the tongue base and soft palate in obstructive sleep apnoea. Acta Otolaryngol. 2004;124:827–832. doi: 10.1080/00016480410017378. [DOI] [PubMed] [Google Scholar]
- e93.Steward DL. Effectiveness of multilevel (tongue and palate) radiofrequency tissue ablation for patients with obstructive sleep apnea syndrome. Laryngoscope. 2004;114:2073–2084. doi: 10.1097/01.mlg.0000149438.35855.af. [DOI] [PubMed] [Google Scholar]
- e94.Friedman M, Lin HC, Gurpinar B, Joseph NJ. Minimally invasive single-stage multilevel treatment for obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2007;117:1859–1863. doi: 10.1097/MLG.0b013e3180f62b4d. [DOI] [PubMed] [Google Scholar]
- e95.Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea and the hyoid: a revised surgical procedure. Otolaryngol Head Neck Surg. 1994;111:717–721. doi: 10.1177/019459989411100604. [DOI] [PubMed] [Google Scholar]
- e96.den Herder C, van Tinteren H, de Vries N. Hyoidthyroidpexia: a surgical treatment for sleep apnea syndrome. Laryngoscope. 2005;115:740–745. doi: 10.1097/01.mlg.0000156464.37681.BF. [DOI] [PubMed] [Google Scholar]
- e97.Stuck BA, Neff W, Hörmann K, et al. Anatomic changes after hyoid suspension for obstructive sleep apnea: an MRI study. Otolaryngol Head Neck Surg. 2005;133:397–402. doi: 10.1016/j.otohns.2005.06.002. [DOI] [PubMed] [Google Scholar]
- e98.Friedman M, Soans R, Gurpinar B, Lin HC, Joseph N. Evaluation of submucosal minimally invasive lingual excision technique for treatment of obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2008;139:378–384. doi: 10.1016/j.otohns.2008.06.011. [DOI] [PubMed] [Google Scholar]
- e99.Fujita S, Woodson BT, Clark JL, Wittig R. Laser midline glossectomy as a treatment for the obstructive sleep apnea. Laryngoscope. 1991;101:805–809. doi: 10.1288/00005537-199108000-00001. [DOI] [PubMed] [Google Scholar]
- e100.Woodson BT, Fujita S. Clinical experience with lingualplasty as part of the treatment of severe obstructive sleep apnea. Otolaryngol Head Neck Surg. 1992;107:40–48. doi: 10.1177/019459989210700107. [DOI] [PubMed] [Google Scholar]
- e101.Mickelson SA, Rosenthal L. Midline glossectomy and epiglottidectomy for obstructive sleep apnea syndrome. Laryngoscope. 1997;107:614–619. doi: 10.1097/00005537-199705000-00011. [DOI] [PubMed] [Google Scholar]
- e102.Robinson S, Lewis R, Norton A, McPeake S. Ultrasound-guided radiofrequency submucosal tongue-base excision for sleep apnoea: a preliminary report. Clin Otolaryngol Allied Sci. 2003;28:341–345. doi: 10.1046/j.1365-2273.2003.00719.x. [DOI] [PubMed] [Google Scholar]
- e103.Friedman M, Soans R, Gurpinar B, Lin HC, Joseph N. Evaluation of submucosal minimally invasive lingual excision technique for treatment of obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2008;139:378–384. doi: 10.1016/j.otohns.2008.06.011. [DOI] [PubMed] [Google Scholar]
- e104.DeRowe A, Günther E, Fibbi A, et al. Tongue-base suspension with a soft tissue-to-bone anchor for obstructive sleep apnea: preliminary clinical results of a new minimally invasive technique. Otolaryngol Head Neck Surg. 2000;122:100–103. doi: 10.1016/S0194-5998(00)70152-5. [DOI] [PubMed] [Google Scholar]
- e105.Woodson BT, deRowe A, Hawke M, et al. Pharyngeal suspension suture with Repose bone screw for obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;122:395–401. doi: 10.1016/S0194-5998(00)70055-6. [DOI] [PubMed] [Google Scholar]
- e106.Woodson BT. A tongue suspension suture for obstructive sleep apnea and snorers. Otolaryngol Head Neck Surg. 2001;124:297–303. doi: 10.1067/mhn.2001.113661. [DOI] [PubMed] [Google Scholar]