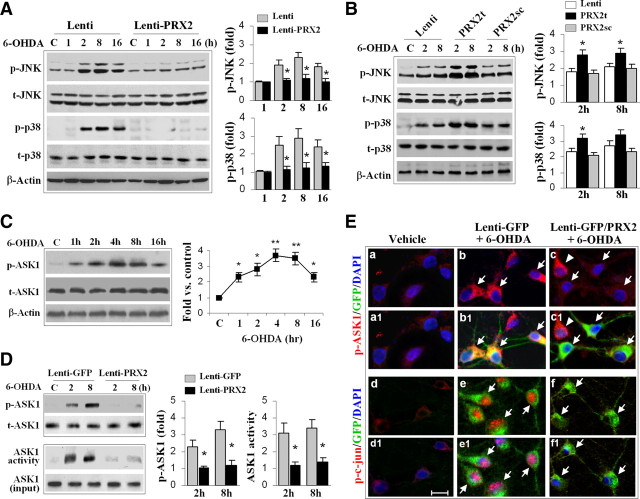
Figure 4.
PRX2 inhibits 6-OHDA-induced activation of ASK1 and JNK/p38 signaling pathways in MN9D cells. A, Neuronal-differentiated MN9D cells were infected with Lenti-PRX2, the empty vector (Lenti), or Lenti-GFP (data not shown) for 3 d, and then challenged with 6-OHDA (50 μm). At the indicated time points, cell extracts were subjected to immunoblotting against p-JNK, total JNK (t-JNK), p-p38, or total p38 (t-p38). The fold changes of p-JNK and p-p38 over the vehicle controls are illustrated in the graphs. *p < 0.05 versus empty vector control (Lenti); data are from three experiments. B, MN9D cells were infected for 3 d with lentiviral vectors containing shRNA PRX2t or PRX2sc, or the empty vector (Lenti), and immunoblotting was performed at 2 and 8 h after 6-OHDA (50 μm). The fold changes of p-JNK and p-p38 over the vehicle controls are illustrated in the graphs. *p < 0.05 versus empty vector control (Lenti) or PRX2sc-infected cells; data are from three experiments. C, 6-OHDA induces ASK1 phosphorylation in MN9D cells in a time-dependent manner. The graph illustrates the temporal profile of p-ASK1 increases after 6-OHDA (50 μm) exposure. *p < 0.05, **p < 0.01 versus vehicle control; data are from three experiments. D, Under the experimental conditions described in A, cell extracts were processed for immunoblotting against p-ASK1 or ASK1 kinase activity assay. The fold changes of p-ASK1 and ASK1 activity over vehicle control are illustrated in the graphs. *p < 0.05 versus Lenti-GFP; data are from three experiments. E, MN9D cells were infected for 3 d with Lenti-GFP or both Lenti-GFP and Lenti-PRX2, and then challenged with 6-OHDA (50 μm). Representative triple-label immunofluorescent images show increased cytosolic p-ASK1 (b, red; b1, yellow, merged with GFP) and nuclear p-c-Jun (e, red; e1, pink, merged with DAPI) immunofluorescence after 6-OHDA; infection with Lenti-PRX2 abolishes the p-ASK1 (c and c1) and p-c-Jun (f and f1) immunofluorescence after 6-OHDA.